Fungi: General Characteristics, Classification, Morphology, Pathogenecity, Sample collection, Lab Diagnosis, Treatment and Prevention

Fungi
Fungi: All medical fungi under a single roof containing outlines general characteristics, classification, morphology, pathogenicity, sample collection, laboratory diagnosis, treatment, and Prevention.
Fungus: Singular
Fungi: Plural
Course Objectives
This course will enable us to become familiar with medically important fungi and to diagnose the infections caused by fungi.
Course Contents
1. Introduction to Mycology
Introduction, Classification of medically important fungi, Fungal species associated with AIDS.
2. Medically Important Fungi
General characteristics of medically important fungi and their significance to human beings, Opportunistic fungi.
3. Specimen Preparation
Procedures for collection and preservation of clinical specimens for diagnostic purposes.
4. General Characteristics, Pathogenesis, Clinical Findings, Laboratory Diagnosis, Epidemiology and Diseases, Prevention and Control of the following Fungi-
- Candida albicans
- Cryptococcus neoformans
- Aspergillus spp.
- Fusarium spp.
- Phialophora spp.
- Trichophyton
- Microsporum
- Epidermphyton spp.
- Sporothrix spp.
- Histoplasma capsulatum
- Blastomyces dermastitidis
- Coccidiodes immitis
- Paracoccidioides brasiliensis
5. Antifungal sensitivity test, Antifungal drugs
Introduction to Mycology
Introduction, Classification of medically important fungi, Fungal species associated with AIDS
Mycology is the study of fungi and the name is derived from Mykos meaning mushroom.
Medical mycology: The science that deals with the study of fungi that causes the disease is called medical mycology.
General Features
- All fungi are eukaryotes
- Natural habitat: soil, water, and decaying organic debris
- Obligate or facultative aerobes
- Chemotrophic organisms and thus obtaining their nutrient from a chemical in nature
- Even being pathogens some fungi are useful to us e.g. edible mushrooms, use of yeasts in the fermentation of fruit juices, and some fungi useful for antibiotic production (Penicillium).
Fungi differ from bacteria due to having the following properties-
- Posses rigid cell wall
- Contain chitin, mannan, and polysaccharide
- The cytoplasmic membrane contains sterols.
- The cytoplasm contains nuclei with nuclear membrane, mitochondria, and endoplasmic reticulum
- Unicellular or multicellular
- Divide by asexually, sexually, or by both.
Classification
Classification: It is of two types-
- Morphological Classification
- Taxonomical classification
Morphological classification: It is of the following types-
a. Yeast
b. Yeast like fungi
c. Mould
d. Dimorphic Fungi
Yeast
- Round to oval unicellular
- Reproduce by budding
- Creamy mucoid colonies on SDA
- e.g. Cryptococcus neoformans
Yeast like fungi
- Partially as yeast and partially as elongated.
- Germ tube to demonstrate pseudohyphae. e.g. Candida albicans
Molds
- Grow as branching filaments hyphae
- Hyphae septate or aseptate
- Continue growth called mycelium e.g. Dermatophytes, Aspergillus, Penicillium, and Rhizopus.
Dimorphic Fungi
- They show thermal dimorphism.
- They exist as yeasts in host tissue and in culture as mycelial growth e.g.
- Sporothrixi schenckii
- Blastomycosis
- Histoplasma
- Candida albicans
- Paracoccidiodes
- Penicillium
- Coccidioidodes
Remember as: Senior Boys Hostel CA Powerful Personal Computer
Taxonomic classification
Division: Thallophyta: Irregular plant masses lacking definite, root, stem, and leaf structures.
Fungi——————–Alage
-No chlorophyll -chlorophyll
There are four phyla.
Zygomycota
- Lower fungi having usually aseptate hyphae
- Forms asexual spores sporangiospores
- Sexual spores zygospores and oospores
- Broad hyphae only a few with septa
- Only mold forms e.g. Absidia, Mucor, Rhizopus, Rhizomucor, Syncephalastrum, Cunningghamella, Conidiobolus, Basidiobolus.
Ascomycota
- Septate hyphae
- Sexual spores are ascospores and they are present within sac or ascus.
- Both yeasts and molds forms
- Hyphae if present with narrow and regular septa
- Asexual spores conidia e.g. Penicillium, Aspergillus, Pneumocystis, Sporothrix, Fusarium, Acremonium, Cladosporium, Hortaea werneckii, Piedraia hortae, Bipolaris, Exserohilum, Curvularia, Alternaria.
Basidiomycota
- Septate hyphae
- Sexual spores are basidiospores on a basidium
- Asexual spores( propagules)-Conidia
- Both yeast and molds form
- Narrow hyphae with regular septa e.g. Malassezia, Trichosporon, Rhodotorula, Sporotrichum.
Deuteromycetes
- Fungi imperfecti
- Narrow hyphae with regular septa
- Asexual spores-Conidia
- Lack of a known sexual state
- Most medically important fungi belong to this phylum e.g. Acremonium, Saccharomyces, Trichosporon, Malassezia, Aspergillus, Penicillium, Blastomyces, Coccidiodes, Histoplasma, Trichophyton, Sporothrix, Alternaria, Bipolaris, Cladosporium, Nattrassia.
Reproduction and sporulation
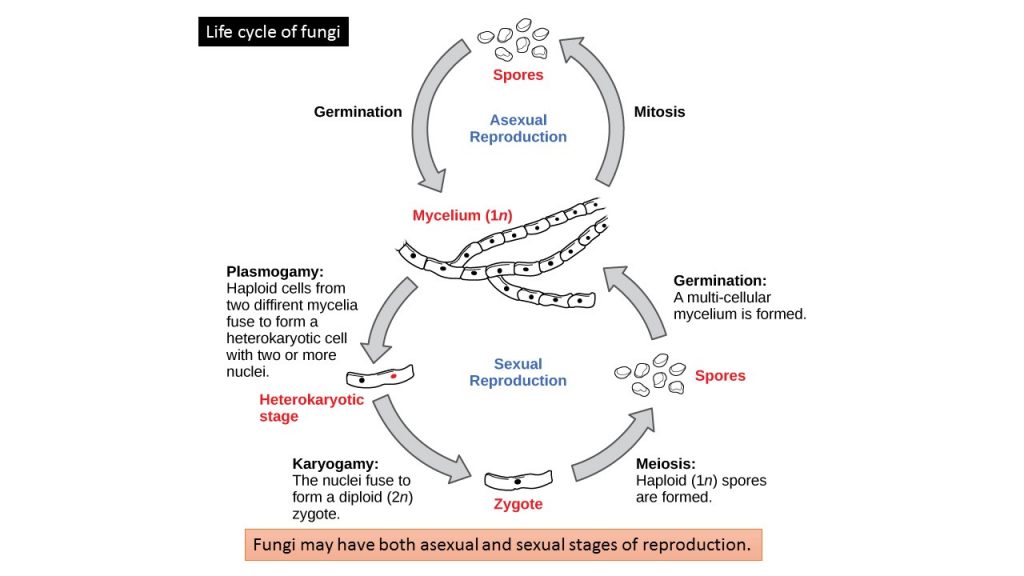
Sexual Reproduction
Sexual reproduction introduces genetic variation into a population of fungi. In fungi, sexual reproduction often occurs in response to adverse environmental conditions. During sexual reproduction, two mating types are produced. When both mating types are present in the same mycelium, it is called homothallic, or self-fertile. Heterothallic mycelia require two different, but compatible, mycelia to reproduce sexually. Sexual spores- Oospores, ascospores, zygospores, and basidiospores
Asexual Reproduction
Fungi reproduce asexually by fragmentation, budding, or producing spores. Fragments of hyphae can grow new colonies. Somatic cells in yeast form buds. During budding (a type of cytokinesis), a bulge forms on the side of the cell, the nucleus divides mitotically, and the bud ultimately detaches itself from the mother cell. Asexual spores: It is of two types, classification based on growth location- Vegetative spores and aerial spores
Vegetative spores: Arthrospores: Cross-septa into hyphae
Blastospores: Forms by budding from the parent cell
Chlamydospores
Aerial spores: Conidiospores, sporangiospores, microconidia, macroconidia
Note: The sexual form is known as teleomorph and the asexual form is known as anamorph.
Classification of fungi on the basis of growth rate
According to growth rate, fungi are classified into three groups and they are-
- Rapid growers: Growth of fungi within 1-5 days. e.g. Candida, Aspergillus
- Intermediate growers: 6-12 days e.g. Sporothrix schenkii
- Slow growers: 13-28 days e.g. Histoplasma capsulatum, Dermatophytes
Classification based on refractive appearance
Hyphae are described as “gloeoplerous” (“gloeohyphae”) if their high refractive index gives them an oily or granular appearance under the microscope. These cells may be yellowish or clear (hyaline). They can sometimes selectively be colored by sulphovanillin or other reagents. The specialized cells termed cystidia can also be gloeoplerous.
The life cycle of fungi
Fungal species associated with AIDS
The most common fungal species which are associated with AIDS patients are given below-
i. Candida albicans
ii. Pneumocuystis jirovecii
iii. Cryptococcus neoformans
iv. Histoplasma capsulatum
v. Coccidiodes immitis
vi. Penicillium marneffei
vii. Aspergillus species
Opportunistic fungal infections such as mucocutaneous candidiasis, pneumocystosis, cryptococcosis, and histoplasmosis are the most common AIDS-defining conditions in HIV- positive individuals. Other fungal infections like coccidioidomycosis and Penicilliosis ( P. marneffei) are usually seen in geographically restricted areas, the latter being reported frequently from northeast India.
Blastomycosis and paracoccidioidomycosis have been reported to cause severe and disseminated disease in AIDS patients, though there has been no significant increase in the number of infections occurring in such patients. Unlike the above diseases, which occur primarily due to the defect in cell-mediated immunity, aspergillosis and zygomycosis are now being increasingly encountered in advanced AIDS cases with neutropenia. Neutropenia in these patients also increases the risk of disseminated candidiasis and invasive infections due to miscellaneous hyaline and dematiaceous (melanized) fungi. In Asia, paracoccidioidomycosis has only been reported from Japan, and only two authentic cases of coccidioidomycosis were reported from India (both were imported cases from an endemic area).
CD4 T lymphocytes and fungal infections
CD4 counts, a useful prognostic marker in HIV/AIDS patients, also have critical levels below in which certain invasive fungal infections start appearing frequently-
CD4 count Opportunistic fungal infection
a. <350 cells/µl – Mucocutaneous candidosis, Pneumocystosis
b. <150 cells/µl – Histoplasmosis
c. <100 cells/µl- Cryptococcosis and Penicilliosis
d. <50 cells/µl- Aspergillosis and zygomycosis
2. Medically Important Fungi
General characteristics of medically important fungi and their significance to human beings, opportunistic fungi.
Classification of Mycoses
Mycosis-Singular
Mycoses-Pleural,
A disease caused by any fungus that invades the tissues, and according to tissue involvement they are of the following types-
superficial, cutaneous, subcutaneous, systemic mycoses, and even opportunistic mycoses.
Superficial mycoses: They are strictly surface infections. e.g. Pityriasis Versicolor- affects stratum corneum of hair ( causative agent- Malassezia furfur)
- Tinea nigra: Black or brownish macular lesions especially of palm ( causative agent- Exophilia werneckii)
- Piedra: Irregular nodules along the hair shaft
- Black Piedra- causative agent Piedra hortae
- White Piedra-Causative agent Trichosporon spp.
Cutaneous Mycoses
- Superficial fungal infections of the skin, hair, or nails.
- No living tissue is invaded,
- Dermatophytosis and Ringworm of the scalp, glabrous skin, and nails-causative agent Trichophyton, Epidermophyton, and Microsporum.
- Species of fungi causing ringworm can be ecologically divided into three groups:
Zoophilic or “animal-loving.” Species infect animals primarily, e.g. cats, dogs, horses, cows.
Anthropophilic or “man loving.” Species infect people and cannot be transferred to animals.
- Geophilic or “earth-loving.” Species occur naturally in soil, presumably as a saprobe, but are capable of
infecting animals and people. In another word, these are facultative parasites.
Candidiasis of skin, mucous membranes, and nails- causative agents are Candida,
Debaryomyces, Kluyveromyces, Meyerozyma, Pichia, etc. Dermatomycosis is rare and caused by Non-
dermatophyte molds, Neoscytalidium and Scopulariopsis.
Ringworm infections are conveniently divided into categories, based on the part of the body
that was infected:
Tinea capitis: Ringworm of the scalp, eyebrow, and lashes.
Tinea corporis: Ringworm of the body.
Tinea cruris: Ringworm of the groin, perineum, and perianal region. Infections are commonly referred to as “jock itch”.
Tinea unguium: Ringworm of the nail.
Tinea barbae: Ringworm of the beard.
Tinea pedis: Ringworm of the feet. Infections are commonly referred to as athlete’s foot.
Tinea manuum: Ringworm of the hand.
Subcutaneous mycoses
These are chronic, localized infections of the skin and subcutaneous tissue following the traumatic implantation of the aetiologic agent. The causative fungi are all soil saprophytes of regional epidemiology whose ability to adapt to the tissue environment and elicit disease is extremely variable.
Mycetoma: Scedosporium, Madurella, Trematosphaeria, Acremonium, Exophiala, etc.
Chromoblastomycosis: Fonsecaea, Phialophora,Cladophialophora etc.
Sporotrichosis: Sporothrix spp.
Rhinosporidiosis: Rhinosporidium seeberi
Phaeohyphomycosis: Cladophialophora, Exophiala,
Bipolaris, Exserohilum etc.
Note: Incidence of subcutaneous mycoses is rare.
Systemic mycoses
Systemic mycoses are fungal infections affecting internal organs. In the right circumstances, the fungi enter the body via the lungs, through the gut, paranasal sinuses, or skin. The fungi can then spread via the bloodstream to multiple organs including the skin, often causing multiple organs to fail and eventually resulting in the death of the patient.
- Histoplasma capsulatum(causing histoplasmosis)
- Coccidioides immitis(causing coccidioidomycosis)
- Blastomyces dermatitidis(causing blastomycosis)
- Paracoccidioides brasiliensis(causing paracoccidioidomycosis)
- Talaromyces marneffei( previously called Penicillium marneffei)-(causing talaromycosis)
Risk factors
- HIV infection
- Systemic malignancy (cancer)
- Neutropenia (low white blood cell count)
- Organ transplant recipients and following hematological stem cell transplant
- After a major surgical operation
- Poorly controlled diabetes mellitus
- Adult-onset immunodeficiency syndrome
- Very old or very young
Opportunistic Mycoses
Those that affect the immunocompromised but are rare in normal individuals.
Organ transplantation, post-chemotherapy for cancer, immunodeficient due to AIDS, and congenital immunodeficiency states.
Candida species are the most commonly occurring fungal pathogen in the ICU setting.
Opportunistic Fungal pathogens are-
- Penicillium
- Candida
- Aspergillus
- Mucor
- Pneumocystis jirovecii ( learn as PCAMP)
- Cryptococcus
- Absidia
Specimen
It depends on the site of infection. e.g. in case of cryptococcal meningitis CSF, infection due to dermatophytes skin scrapping, hair plucking, nail clipping, pulmonary histoplasmosis sputum or Bronchial alveolar lavage(BAL) whereas in vulvovaginal candidiasis vaginal swab, etc.
Laboratory Diagnosis of Fungi
Direct Microscopy: KOH preparation
Gram stain
India ink or Nigrosin preparation
Calcofluor White Stain
Culture:
SDA. PDA, CMA, Czapek-Dox agar, DTM, Birdseed agar (BSA), slide culture
LPCB preparation:
Serological Tests
Special stains: Giemsa, PAS, and Grocott methamine silver stain
Molecular Tests-PCR
Allergic skin test with trichophytin, candidin
Note: Clinical examination-Wood’s Lamp for screening fungal infections
Differences between true and opportunistic fungal infection-
True pathogens are of four genera-
- Histoplasma
- Blastomyces dermatitidis
- Coccidioides immitis
- Paracoccidioidesbrasiliensis
Opportunistic pathogens are-
- Candida
- Aspergillus
- Pneumocystis jirovecii
- Mucor
- Penicillium
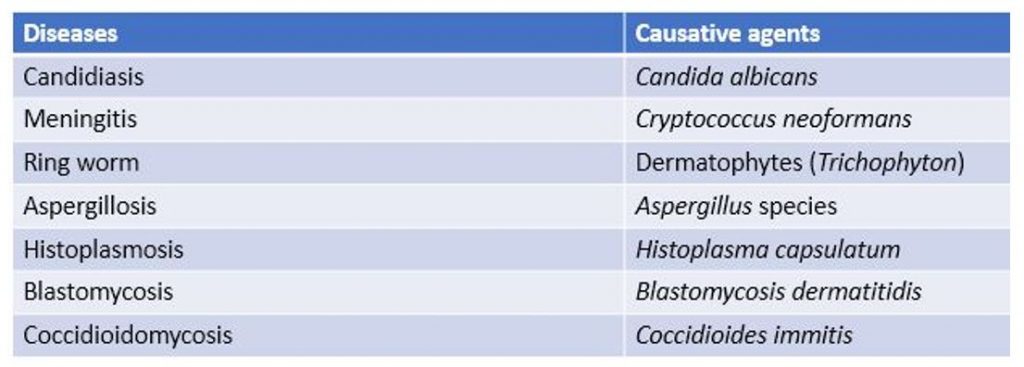
Some fungal diseases and their causative agents-
Predisposing factors of fungal infection
- Diabetes
- Prolonged treatment with corticosteroids
- Immunosuppression
- Broad antibiotic therapy
- Injury
Prevention and control of fungal infection
Fungal infections result from direct invasion of tissue and organs or direct inhalation of fungal spores or their hyphae. Most infections are acquired through exposure. So, the fungal disease can be prevented by applying the measures that prevent or reduce exposure to the fungi and controlled by proper treatment of cases.
Preventive measures are as follows-
- Improvement of sanitary facilities e.g. improving living conditions i.e. hosting, flooding, and proper nutrition.
- Personal protection e.g. hand and foot protection while working on the field covering the site where any cut or scratch is found, wearing a mask while working in an old building or area where fungal spores are readily found.
- Environmental management e.g. proper disposal of decaying vegetation, removal of bird droppings, rotten woods, and so on from the living site.
- Improvement of health care facilities for example proper diagnosis and care facilities, early diagnosis, and treatment of cases.
- Reduction of predisposing factors that insist on infection e.g. reduce broad antibiotic therapy, reduce prolonged treatment with a corticosteroid, reduce stress, etc.
Treatment of fungal infections/ diseases
Fungal diseases are treated by anti-fungal agents and those agents are categorized into three-
- Polyenes e.g. Amphotericin B, nystatin, griseofulvin
- Azoles e.g. clotrimazole, ketoconazole, fluconazole, miconazole, itraconazole, voriconazole
- Nucleoside derivatives e.g. 5-fluorocytosine
Notes:
Most of these drugs are fungistatic except a few like amphotericin B. allylamine, benzylamine, and morpholines, which are fungicidal.
Nystatin is the first discovered antifungal drug in 1951 and abbreviated for New York State Institute. They can also be classified as topical or systemic antifungal agents and on the basis of their route of administration.
Unit 3: Specimen Preparation
Procedures for collection and preservation of clinical specimens for diagnostic purposes.
Safety considerations
Specimen collection- Universal precautions should be followed
Specimen transport/storage- Sealed plastic bag (preferably double-layered) with proper legible ( clear enough to read) labeling
Specimen processing- Containment level II or III lab
Specimen disposal- All infected material should be treated as per biomedical waste management guidelines
Specimen collection
The optimum time of specimen collection: Ideally as close to the onset of symptoms as feasible
- Before initiation of antifungal therapy, where possible
- Morning sample for inpatients
Correct specimen type: · Appropriate specimen according to the site of the lesion, inadequate amount (as mentioned below), collected with sterile implements and precautions
CSF > 2 ml
- Sterile body fluids (pleural, pericardial, synovial, ascitic fluid) > 10 ml
- Lower respiratory (BAL, bronchoscopic washings/aspirate) > 1 ml (ensure
it contains lower respiratory tract specimen)
- Upper respiratory – oral swab/saline wash
- Nasal sinuses – surgical removal of sinus contents
- Urine (midstream) 10-20 ml (for catheter urine, clamp hub of Foley’s catheter distally, clean hub sequentially with 70% alcohol, iodine, and 70% alcohol, then aspirate collected urine from the hub with sterile needle and syringe)
Hair, Skin, and nail
- The scalps of patients with suspected tinea capitis may be examined with a Wood’s lamp. Fluorescent distorted or fractured hairs should be removed with forceps. Infected hairs can easily be removed, but normal hairs are more difficult to dislodge. A comb or brush may be used to collect loose hair and skin squames.
- Skin, when involved, should be cleansed with an alcohol wipe before a specimen is collected. Epidermal scales at the active border of a lesion should be removed with a scalpel. Nails should be cleansed with an alcohol wipe, and the outermost layer should then be removed by scraping with a scalpel.
- Deeper scrapings, debris from under the edges of the infected nails, and nail clippings from infected areas are also suitable for culture.
- Samples of hair, skin, and nails should be collected and placed in a sterile culture dish for transport to the Laboratory. Storage of 4°C is not recommended since at least one dermatophyte is susceptible to cold temperatures. In addition, storage in closed containers is unsuitable due to the overgrowth of contaminating bacteria and saprobic fungi in a moist environment.
- Nail clippings may be ground in a mortar before being inoculated onto culture media. Skin scrapings and hair may be inoculated directly onto the surface of appropriate culture media.
Sputum 2-5 ml
- Coughed-out sputum (not saliva) in a wide-mouthed sterile container; Q score is calculated from a
gram stained smear and only representative samples are accepted
– In case of dry cough, induced sputum in a sterile container
- Stool 1-5 g (rarely used, only to know colonization by Candida)
Vaginal Swab
Prostatic Fluid – Bladder emptied followed by prostatic massage
Blood
- Blood 20 ml-10 ml in each bottle; for pediatric patients 4-10 ml divided equally between two bottles
- Candidemia is known to be intermittent, so it is essential to obtain three samples to rule out yeast sepsis
- Inoculated directly to biphasic blood culture bottle maintaining a ratio of 1:10 of blood to the broth
- Serum – additional 2-5 ml of blood to be put in clean, dry vials, preferably leak-proof screw-capped for serum separation
Subcutaneous sites
Abscess – Aspirate with sterile needle and syringe, if needed
sample base of lesion and abscess wall (scrape, punch biopsy)
Open wound – Aspirate or swab deeply, especially base and margins
Tissue biopsy specimen – surgical collection, punch biopsies may be used for skin lesions. Repeated sampling from the same site (for authentic diagnosis of opportunistic infection repeated demonstration/isolation of the same organism from the same site is essential)
Bone Marrow
As with the collection of other sterile body fluids, good skin antisepsis should be practiced for bone marrow sample collection. 10 ml of bone marrow should be collected at periodic intervals as determined by the physician.
Bone marrow is inoculated to Blood Culture bottles at the bedside. Bottles should be returned immediately to the laboratory for incubation at 35°C for 28 days.
Notes:
- All efforts should be made to collect specimens for fungal culture as free from bacterial contamination as possible.
- CSF should NOT be refrigerated, since it is an excellent culture medium and fungi will continue to replicate at 25- 30°C.
Specimen transport and storage
- The time between specimen collection and transport: Specimens should be transported to the specific laboratory as soon as possible · Maximum time allowed for transport is 24 hours at room temperature from the sterile site; specimen from the non-sterile site may be transported at 4°C if the time for transport is > 1 hour.
- Storage: Specimen should be processed in the laboratory as soon as possible. Delay of more than four hours in the processing of unrefrigerated specimens is undesirable. Where there is a delay in processing, specimens should be refrigerated except CSF and specimens for isolation of Cryptococcus·
- As a general principle, sterile samples should never be refrigerated, while samples expected to contain commensal should be refrigerated if there is a delay.
Unit 4. General Characteristics, Pathogenesis, Clinical Findings, Laboratory Diagnosis, Epidemiology and Diseases, Prevention and Control of the following Fungi:
Aspergillus spp., Candida albicans, Fusarium spp., Cryptococcus neoformans, Histoplasma capsulatum, Sporothrix spp., Philophora spp., Trichophyton, Microsprum, Epidermphyton spp., Blastomyces dermastitidis, Coccidiodes immitis, Paracoccidioides brasiliensis.
Candida albicans: Introduction, Morphology, Pathogenicity, Laboratory Diagnosis, and Treatment
Introduction of Candida
The yeast is a common commensal of the gastrointestinal tract. Most Candida species are opportunistically occurring in debilitated persons e.g. Diabetes patients, those who are taking anticancer therapy and immunocompromised patients, HIV patients, and so on.
Classification of Candida
Kingdom: Fungi
Division: Ascomycota
Class: Saccharomycetes
Order: Saccharomycetales
Family: Saccharomycetaceae
Genus: Candida
Species: C. albicans
Other medically important Candida species are Candida albicans, Candida tropicalis, Candida parapsilosis, Candida glabrata and Candida krusei.
Morphology of Candida
Yeasts are small, oval, measuring 3-4 micrometers in diameter. Single, budding of the cells may be seen. The yeast cells can also be seen attached with pseudohyphae.
Pathogenicity of Candida albicans
It causes a disease called candidiasis also called moniliasis. It is an infection causing fungi of the genus
formerly Monilia or now Candida (especially Candida albicans).
The various forms of diseases are –
Oral thrush: Also known as Candidiasis of the mouth or oropharyngeal candidiasis which is seen as white patches on the mucosa of the mouth including the tongue. The affected site can become inflamed and may cause difficulty in swallowing causing cracking and inflammation which may occur around the mouth. Such a condition is referred to as oral cheilitis. Oral thrush may spread to the esophagus (esophagitis). Although most people harbor Candida species, oral candidiasis is typically found in immunocompromised hosts like AIDS patients (9 to 31% ), persons taking immunosuppressive drugs for cancer chemotherapy (20%), and organ transplantation. Other factors associated with oral thrush are diabetes, certain dentures, and the use of corticosteroids.
5 and 7% of neonates develop oral candidiasis( CDC) and untreated oral thrush can lead to serious invasive disease.
Vaginal thrush: Also called genital or vulvovaginal candidiasis causes genital itching, a burning sensation, and vaginal discharge in females, and while In men, the penis may have an itching rash. This is rare in men but most women will have at least one episode of vulvovaginal candidiasis. Women with the following conditions are more at risk of the infection if they are-
√Pregnant
√Diabetic
√Use broad-spectrum antibiotics
√Use corticosteroids
Leucorrhoea: It is a flow of a whitish, yellowish, or greenish discharge from the vagina that may be normal or a sign of infection. Discharges may originate from the various female reproductive parts such as the vagina, ovaries, fallopian tubes, or, most commonly, the cervix. Leukorrhea may occur during pregnancy and is considered normal when the discharge is thin, white, and relatively odorless. Physiologic leukorrhea is a normal condition occurring within several months to a year of the onset of the menstrual cycle in adolescent girls and is sometimes present in newborn girls, usually lasting one to two months. However, in many cases, leukorrhea is a sign of infection, especially when the discharge is yellow or green, with an offensive odor, and is accompanied by irritation, itching, pain, or tissue inflammation due to Candida.
Candidemia: Also called Invasive candidiasis is a serious disease when Candida, which is normally on the skin or the gastrointestinal tract (GIT), enters the bloodstream where it can disseminate to other organs. Patient with such condition has symptoms like fever and chills that do not respond to antibacterial agents. These are often nosocomial ( hospital-acquired) infections of people who:√have a central venous catheter√are immunosuppressed√take broad-spectrum antibiotics√show neutropenia√are on hemodialysis√have diabetes
Meningitis and meningoencephalitis: Meningitis due to Candida is mucocutaneous and deeply Invasive Candidiasis are uncommon. Infection can be secondary to hematogenous dissemination or direct inoculation. Neurosurgery, recent antibiotics, and corticosteroids are predisposing factors. Fever, meningismus, elevated CSF pressures, and localizing neurologic signs are commonly noted.
Vaginitis particularly during pregnancy
Laboratory Diagnosis
Sample collection: Samples are collected according to the site of infections. They may be-
- vaginal swab
- Tongue swab
- Blood
- CSF
- Tissue
- Urine
- Exudate
- Swabs from the mucosal surface
Direct microscopic examination
Wet mount preparation
Gram stain
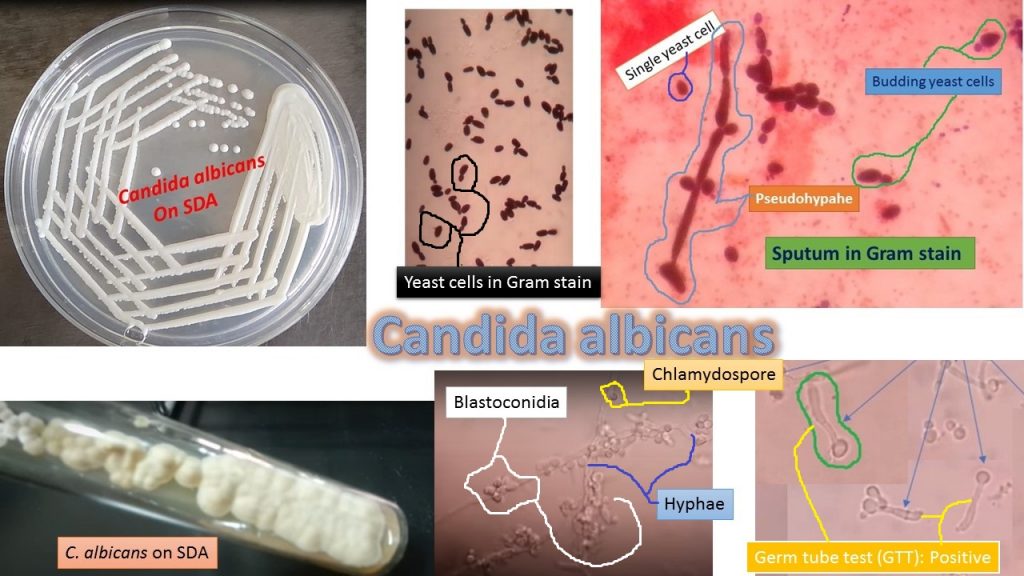
Culture: Culture on Sabouraud Dextrose agar (SDA) at 37° C for 24-48 hours. After incubation observes colonial morphology.
Colony characteristics
Cream-colored pasty and glistening as shown above picture.
Identification of Candida albicans
Wet mount preparation: Single or budding yeast with or without pseudohyphae
Gram stain: single or budding yeast cells with or without pseudohyphae and gram-positive
Germ tube test: Positive
The test is carried out using 0.5 ml rabbit or human serum in which test yeast cells are inoculated and incubated at 37°C for 2-3 hours.
Put a drop of this after 2-3 hours incubation on the slide and cover with the coverslip. Focus at 10X objective and finally observe at high power objective (40X) of a compound microscope.
Result Interpretation of GTT
Germ tube test (GTT) positive: Presence of sprouting yeast cells (as shown above image)
Germ tube test negative: Absence of sprouting yeast cells
Chlamydospore formation: It forms in Cornmeal tween agar after incubation 48-72 hours at 22-25°C. Chlamydospores are spherical, thick-walled, and usually produced on supporting cells that occur along pseudohyphae or at the tip of hyphae as shown above image.
Treatment
Treatment of Candidiasis depends on location and severity.
For oral thrush–Oral nystatin suspension
Similarly for skin and vulvovaginitis –topical antifungals while in resistant case
azole antifungal medication
In severe infections
Amphotericin B
azole antifungals
Echinocandins like micafungin
Keynotes
- All fungi are gram-positive.
- To diagnose Moniliasis, serological tests in patient serum to detect the antibody to Candida albicans should perform. Four folds rise in titer of antibody in paired sera of the patient is diagnostic.
- CHROMagar Candida or HiCrome candida differential agar recommendation is for rapid isolation and identification of Candida species from mixed cultures in clinical and non-clinical samples.
- For Candida spp. identification of other physiological tests like sugar (glucose, galactose, sucrose, maltose, lactose, trehalose) fermentation and assimilation tests are used.
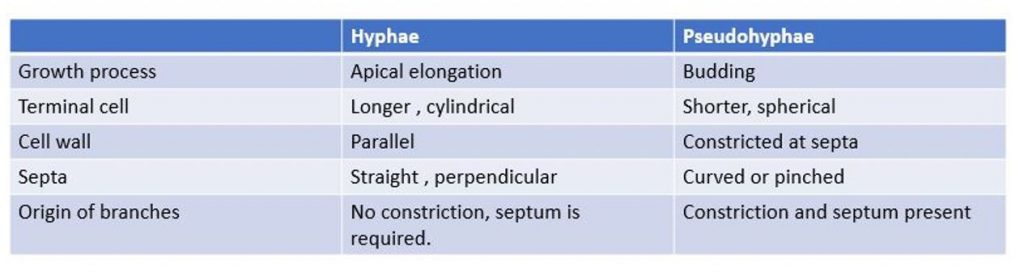
- Differences between hyphae and pseudohyphae are in a table.
Cryptococcus neoformans: Introduction, Pathogenesis, Laboratory Diagnosis, Treatment, Prevention, and Control
Introduction of Cryptococcosis
Cryptococcosis is an acute, subacute, or chronic pulmonary meningeal mycosis caused by Cryptococcus neoformans.
It is a soil saprophyte and is particularly abundant in the feces of pigeons( pigeon’s dropping).
It does not appear to infect birds, probably because of their high body temperature and infection throughout the world.
Classification of Cryptococcus
Kingdom: Fung
Phylum: Basidiomycota
Class: Tremellomycetes
Order: Tremellales
Family: Tremellaceae
Genus: Cryptococcus
Species: C. neoformans
Other species are-
- C. albidus( nitrate positive)
- C. laurentii( melibiose fermentation)
- C. gattii( Trehalose positive)
- C. neoformans: All are negative
- C. neoformansvar.grubii ( serotype A)- worldwide distribution
- C. neoformansvar. neoformans (serotype D)-Restricted distribution and prevalent in France, Italy, and Denmark.
Morphology of Cryptococcus
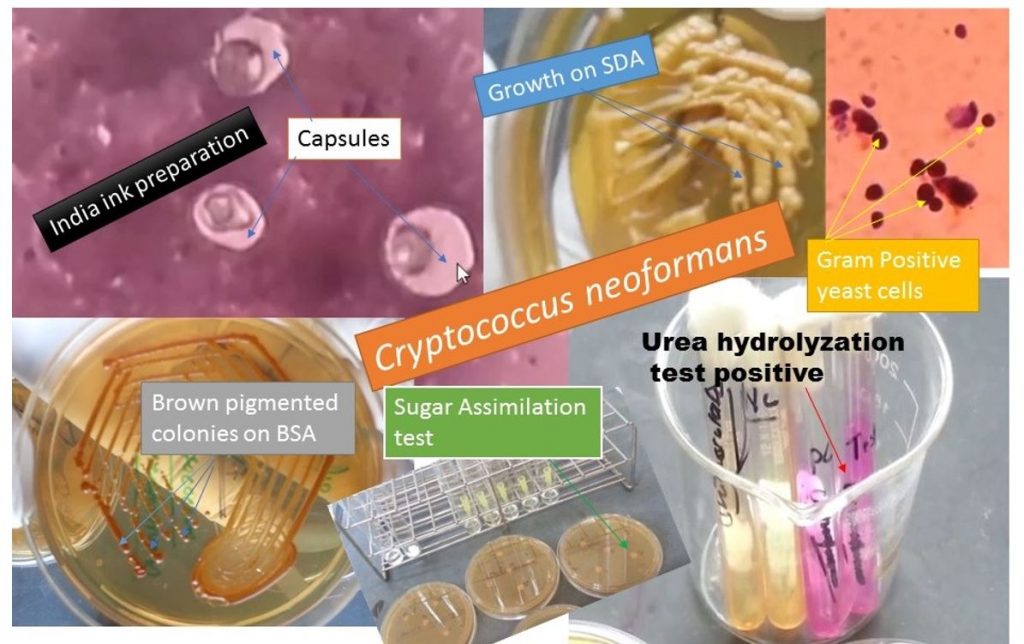
It is a yeast characterized by a wide polysaccharide capsule and budding, found both in culture and tissue fluid. It is a true yeast and is Gram-positive. The capsule may be identified by India ink or nigrosin preparation.
Size
5-15 µm
Antigenicity
Based on carbohydrate antigen, there are 4 serotypes e.g. A, B, C, and D. Infection due to serotypes A and B are common.
Virulence markers
Polysaccharide capsule
Phenol oxidase enzyme
Growth rate at 37°C
Source of infection
Infection is usually acquired by inhalation of dust-containing yeast cells.
Pathogenesis
The disease is usually seen in immunosuppressed hosts and most infections are asymptomatic.
Pulmonary cryptococcosis may lead to mild pneumonitis.
Cryptococcal meningitis happens by hematogenous spread and is often seen in AIDS patients.
Skin, lymph nodes, bones, and other organs may be involved when the dissemination of infection takes place. Cutaneous cryptococcosis varies from small to ulcers to large granuloma.
Visceral forms of cryptococcal infection stimulate tuberculosis and cancer clinically.
Laboratory Diagnosis
Specimens:
- CSF
- Sputum
- pus
- Brain tissue
Direct Microscopy
India ink preparation
Positive due to having capsule appearing as a clear halo around the yeast cells as shown above figure.
Gram stain: Gram-positive yeast cells as shown above figure.
The histopathological examination of tissue can be done by staining with H/E, PAS, and mucicarmine stains.
Culture
Growth on Sabouraud Dextrose Agar (SDA) shows smooth, mucoid, and cream-colored colonies as shown above figure.
Lactophenol cotton blue mount shows budding yeast cells as shown below.
Birdseed agar (BSA): It is a selective medium for Cryptococcus. C. neoformans produces brown colonies within a week at 30°C, such property is not shown by other yeasts. Cryptococcus neoformans produces phenoloxidase, which oxidizes the caffeic acid in the niger seed(resemble sunflower seeds in shape but are smaller in size and black that contains proteins, oil, and soluble sugars, botanical name-Guizotia abyssinica) into melanin.
i.e. Phenol oxidase
↓
caffeic acid………….Brown pigment
Latex agglutination test
Capsular polysaccharide antigen may be detected in CSF, serum, or even in urine by this latex agglutination test.
Animal Inoculation test
C. neoformans inoculation into mice through intracerebral or intraperitoneal routes creates a fatal infection. Encapsulated budding yeast cells can be demonstrated in the brain of the infected mice.
Urea Hydrlolization Test
It shows the urea hydrolyzation test is positive.
Sugar fermentation and assimilation test
They do not ferment carbohydrates.
Molecular Test
Detection of C. neoformans DNA in tissue samples by Nested and Real-Time PCR Assays
Treatment of Cryptococcosis
- Amphotericin B
- Flucytosine
- Ketoconazole
Prevention and Control
- People having Immunocompromized or Immunosuppressed status should avoid contact with birds and avoid digging and dusty activities in areas heavily contaminated with bird droppings.
- Avoid the area where the availability of dried pigeon feces.
- Wear masks to prevent the inhalation of C. neoformans.
Aspergillus: Introduction, Morphology, Pathogenicity, Laboratory Diagnosis, and Treatment
The genus, Aspergillus has more than 180 species, among them, 38 are responsible to cause disease (able to grow at 37°C. They are common in the environment. Aspergillus species are emerging pathogens that are ubiquitous molds that infect immunocompetent ( rarely) and immunocompromised patients ( mainly). The symptoms are diverse and range from allergic reactions, bronchopulmonary infection, and bronchitis, to invasive aspergillosis. A. fumigatus is the main opportunistic pathogen. Other medically important species are Aspergillus niger, Aspergillus flavus, Aspergillus terreus, and Aspergillus nidulans.
Rate of growth
- Usually rapid
- Mature within 3 days
- Only a few species are slower growing
Scientific classification
Kingdom: Fungi
Division: Ascomycota
Class: Eurotiomycetes
Order: Eurotiales
Family: Trichocomaceae
Genus: Aspergillus
Species: Aspergillus fumigatus
Colony morphology
- The surface at first white than any shade of yellow, green, brown, or black depending on Species
- Texture velvety and cottony
- The reverse is white, golden, or brown
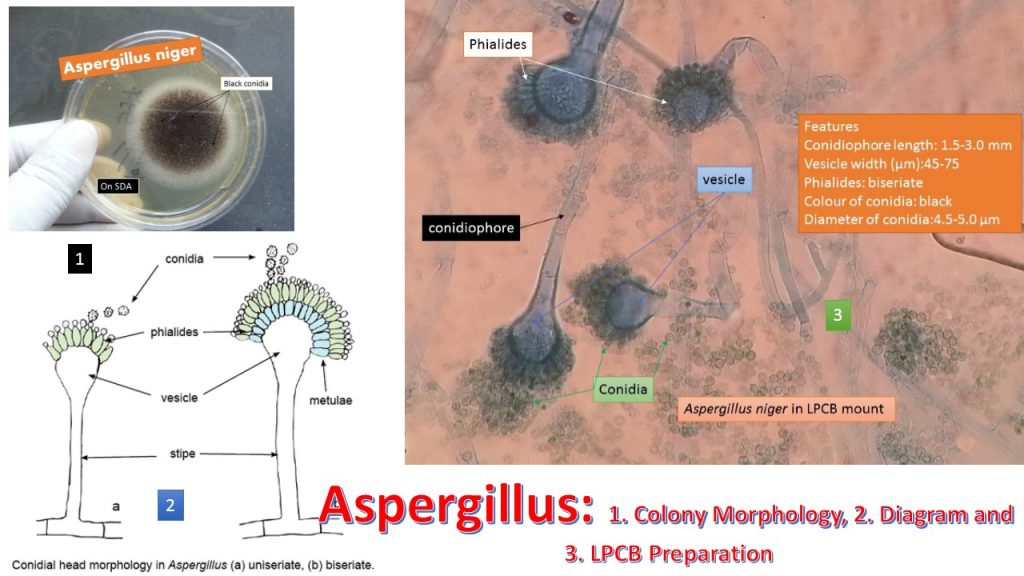
Microscopic morphology
Septate hyphae(2.5-8.0 µm)
Unbranched conidiophore arises from foot cells
The conidiophore is enlarged at the tip forming vesicle
Vesicles are completely or partially covered with flask-shaped phialides
Phialides may develop directly on the vesicle(Uniseriate form) or be supported by metula (biseriate form)
The phialides produce a chain of round conidia(2-5 µm)
The genus Aspergillus – importance to humanity
On the negative side
They cause invasive and allergic diseases in humans and other animals. e.g. A. fumigatus
They cause plant and food spoilage and produce mycotoxins. e.g. A. flavus and A. parasiticus
On the positive side:
Composting: Well-established model organism in cell biology and genetics: A. nidulans
food production:
enzymes and organic acids: A. niger
East Asian foods: A. oryzae and A. sojae
pharmaceuticals:
echinocandins: A. nidulans and A. sydowi
lovastatin: A. terreus
fumagillin: A. fumigatus
Life Cycle
Pathogenesis
Aspergillosis is caused by inhalation of conidia or mycelial filaments which are present on the decaying matter, soil or air. When the host defense is compromised, aspergillosis may develop. The common clinical forms of systemic aspergillosis are as follows-
Respiratory Disease
Bronchopulmonary aspergillosis: The organism grows within the lumen of the bronchioles, which may be occluded by fungus plugs. Some patients may expectorate mucus plugs containing fungus.
Aspergillus asthma: Allergy to aspergilla may occur in atopic individuals following inhalation of spores of aspergilli.
Aspergilloma: It is also known as fungus ball. The fungus colonies in the pre-existing pulmonary cavities such as in tuberculosis or cystic disease.
Invasive aspergillosis
It is also called disseminated aspergillosis and it occurs in severely immunocompromised hosts. The organism first establishes in lung tissue and then disseminates to involve other organs particularly the brain, kidney, and heart.
Superficial infections
Sinusitis: Inflammation of sinus and causative agents are A. flavus and A. fumigatus.
Mycotic Keratitis: Causative agents are A. flavus and A. fumagatus.
Otomycosis: Mainly Aspergillus niger
Immunosuppression and infection
- Inhalation of Aspergillus spores is a common daily occurrence. A healthy immune system would normally remove the spores and no symptoms or infection would occur.
- In individuals whose immune system may be suppressed either because of illness e.g. AIDS, cancer, diabetic patients, or drugs, spores may germinate, and resulting tissue or systemic aspergillus invasion can result.
- Individuals with allergies such as asthma can also be vulnerable to aspergillus disease.
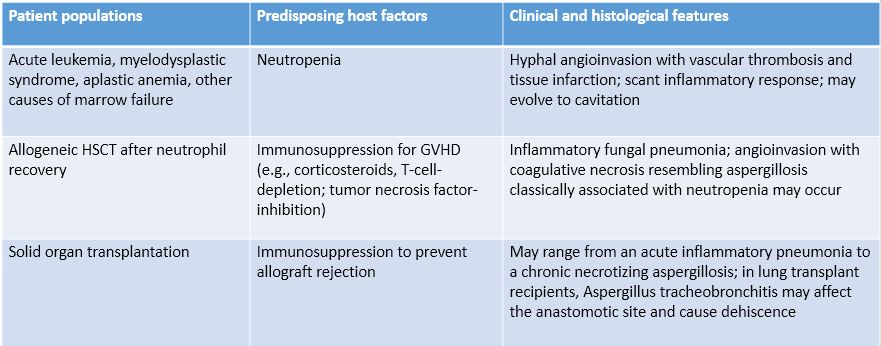
Predisposing host factors and immunopathogenesis of invasive, saprophytic, and allergic bronchopulmonary aspergillosis
Early diagnosis of invasive aspergillosis is important because of-
Mortality 40% 90
Treatment started <10d >11d
-Von Eiff et al, Respiration 1995;62:241-7
Clinical Findings
- Invasive aspergillosis principally involves the sinopulmonary tract, a reflection of inhalation being the most common route of entry of Aspergillus spores (rarely, other sites of entry, such as the gastrointestinal tract or skin, occur).
- Fever, cough, and dyspnea are frequent, although non-specific, findings of pulmonary aspergillosis, the most common site of invasive aspergillosis.
- The vascular invasion may manifest as pleuritic chest pain due to pulmonary infarction or hemoptysis.
- Central nervous system involvement is a devastating consequence of disseminated aspergillosis and may manifest with seizures or focal neurological signs from mass effects or stroke.
- Premature neonates can also develop aspergillosis, with the skin being the most common site of disease.
Laboratory Diagnosis
Specimens
Sputum
Sinus drainage
Bronchial washing
Bronchoalveolar lavage
Biopsy
Direct Microscopy:
Wet mount preparation
KOH Preparation: It shows non-pigmented septate hyphae ( 3-5 µm in diameter) with characteristics of dichotomous branching (ay an angle of approximately 45º)
Biopsy sections can be stained with H &E, PAS, GMS, Acridine orange staining and examined for the characteristics of hyphae.
Culture: The clinical sample is inoculated on two SDA ( lacking cycloheximide) plates and incubated at 37 and 25 ºC respectively.
Generally, colonies appear within 1-3 days and show a velvety to the powdery surface with colors.
Aspergillus fumigatus: green colonies
A. niger: black colonies
A .flavus: golden yellow colonies
Phenotypic identification: It is based on growth characteristics and morphology.
LPCB Preparation: Preparation of colonies shows branching and septate hyphae. Asexual conidia are arranged in chains, carried on sterigmata, borne on the expanded ends called vesicles of conidiophores.
PCR-based diagnosis of invasive fungal diseases, although promising, is currently investigational. Potential advantages include rapidity, low cost, the ability to establish a diagnosis at the species level, and to detect genes that confer antifungal resistance. Limitations include lack of standardized methods, difficulty in reliably distinguishing fungal colonization from disease, and the potential for contamination with fungal DNA.
Skin test
Agar gel diffusion
Aspergilloma: 3-4 bands (100%)
ABPA: 1-3 bands (50-75 %)
Asthma: 1 band ( 50%)
Treatment
Voriconazole has become the gold standard as primary therapy for invasive aspergillosis.
Antifungal agents used to prevent and treat aspergillosis are as follows-
- Voriconazole
- Itraconazole
- Posaconazole
- Amphotericin B deoxycholate
- Liposomal amphotericin B
- Amphotericin B lipid complex
- Amphotericin B colloidal dispersion (ABCD)
- Caspofungin
- Micafungin
- Anidulafungin (no FDA approved dose as therapy for aspergillosis)
Prevention and Control
- Due to the prevalence of aspergillus mold in the environment, it is very difficult to avoid exposure.
- It is best to avoid locations with excessive amounts of dust or mold, such as construction sites or compost piles.
- People with weakened immune systems or mold allergies should avoid activities such as gardening or lawn mowing.
- If exposure to airborne dust or mold is likely, considering wearing a face mask or N95 mask.
- In some cases, your clinician might recommend the use of antifungal medication to prevent infection.
Key Notes
- Thrombocytopenia may limit the ability to perform invasive procedures.
- In contrast to adults, children with invasive pulmonary aspergillosis frequently do not manifest cavitation or the air crescent or halo signs.
- Culture correlation with KOH mount examination is important because aspergilli are common laboratory contaminants.
- 6 consecutive morning sputum samples are required out of which should show the same fungi in 50% of samples.
Fusarium species: General Characteristics, Pathogenesis, Clinical Findings, Laboratory Diagnosis, Treatment, Prevention, and Control
General Characteristics of Fusarium species
Fusarium species are a large group of filamentous fungi belonging to the hyphomycetes. They are commonly distributed in the soil they are saprophytic fungi and are plant pathogens and thus causing a wide range of plant diseases. This is because of their ability to produce mycotoxins especially in cereal crops, that can cause disease in human and animal hosts if ingested. Fusarium species majorly produce fumonisins and trichothecenes mycotoxins. Infections due to Fusarium species are collectively referred to as fusariosis. Fusarium species do not commonly cause diseases in humans because some exist as commensals in the skin, but it has been found to cause opportunistic infections in immunocompromised individuals with clinical manifestation of
- Endophthalmitis
- Sinusitis
- Pneumonia
- Skin Involvement
- Fungemia
- Disseminated Infection
- Fusarium is one of the emerging causes of opportunistic mycoses.
Whereas in pant, plant disease areas follows-Fusarium head blight (FHB, Footrot (FR), root rot (RR), crown rot (CR), Fusarium wilts, Pokkah Boeing on sugarcane and banana disease of rice.
Animal diseases are equine leukoencephalomalacia (ELEM): It is a disease of the central nervous system that affects horses, mules, and donkeys and is also called “Moldy Corn Poisoning”. Abdominal distress, diarrhea, cardiac insufficiency, emesis, and even death in pigs due to consumption of myotoxin. Medically Important Fusarium species are Fusarium solani is the most frequent species, accounting for about 50% of all infections, followed by F. oxysporum (~20%), F. verticillioidis and F. moniliforme. Other species include Fusarium dimerum, F. proliferatum, F. chlamidosporum, F. sacchari, F. nygamai, F. napiforme, F. antophilum and F. vasinfectum.
Scientific Classification of Fusarium species
Kingdom: Fungi
Division: Ascomycota
Class: Sordariomycetes
Order: Hypocreales
Family: Nectriaceae
Genus: Fusarium
Species: F. solani
Habitat of Fusarium species
Fusarium species are commonly found in soil and environmental habitats, with many growing and thriving in tropical and temperate regions and even in desert regions, the alpine, the arctic regions with harch cold conditions, they seem to prevail. It is also found in normal mycoflora of commodities, such as rice, bean, soybean, and other crops. Due to wide distribution and having efficient dispersal mechanisms, they became able to grow in a wide range of substrates as well.
Morphology of Fusarium species
Fusarium species reproduce asexually and produces three kinds of fungal spores known as macroconidia, microconidia, and chlamydospores. Some species of Fusarium produce all three types of spore while others produce singularly. These spores especially the microconidia (2-4 x 4-8 µm) are held by microconidiophores. These conidiophores may be either mono-phialides only or both mono-phialides and poly-phialides in a given species producing microconidia. Macroconidia (3-8 x 11-70 µm) are produced in a sporodochium, which is an erumpent crowded cluster of conidiophores arising from stroma to form a cushion-like mass that supports the macroconidia. These macroconidia are also produced on mono-phialides (a conidiophore with a single opening through which an endoconidia is released) and poly-phialides (two or more openings or pores from which the endoconidia are forced out) on aerial mycelium. The macroconidia vary in size and shape. The Major producers of macroconidia are F. semitectum, F. avenaceum and F. suglutinans. Microconidia are produced in the aerial mycelium. The microconidia can be produced on false heads or false chains on mono-phialides or poly-phialides. False Heads are a result of moisture drops on the conidiophore and they contain the endoconidia as they are produced. Microconidia have different shapes and sizes. The microconidia produced in chains have a truncate base. Chlamydospores are thick-walled spores filled with lipid-like material that carries the spores overwinter in the soil. Chlamydopsores are sometimes airborne occurring in pairs, in clumps, or in chains. They have an outer wall that can be smooth or rough.
Cultural Characteristics of Fusarium species
Sabouraud Dextrose Agar (SDA)
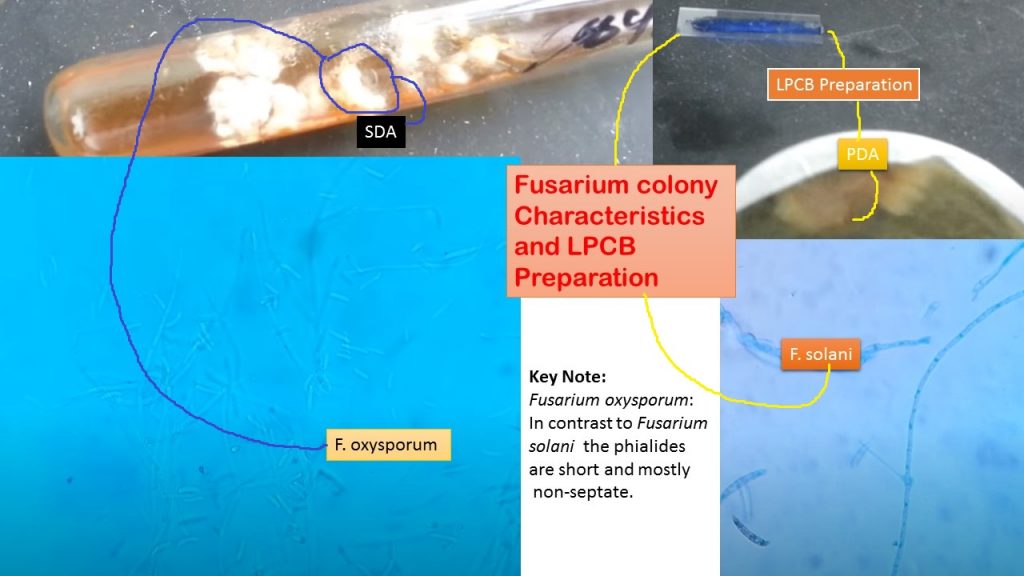
Fusarium spp. grow rapidly on Sabouraud dextrose agar at 25°C and produce woolly to cottony, flat, spreading colonies. The only slow-growing species is F. dimerum. From the front, the color of the colony may be white, cream, tan, salmon, cinnamon, yellow, red, violet, pink, or purple. From the reverse, it may be colorless, tan, red, dark purple, or brown.
Carnation Leaf Agar
Carnation Leaf Agar promotes sporulation and suppresses mycelial growth. It produces conidia and conidiophores in large numbers and specialized morphologies of the spores are distinct. Carnation leaf agar has low carbohydrates with complex substances that provide a natural environment that promotes Fusarium growth.
Potato dextrose agar (PDA)
This is the most valuable medium for Fusarium growth-producing gross morphological appearance and colony colorations. The medium contains a high carbohydrate content which promotes sporulation, however, takes longer to grow in this medium. The conidia produced are misshapen and atypical. The colonies have a velvety or cottony surface, and are white, yellow, pink, purple salmon, or gray on the surface, with a pale, red, violet, brown, or sometimes the blue reverse.
Potassium Chloride Agar (KCLA)
KCLA is used to observe the formation of microconidia in chains by species. The species that form chains of microconidia form more abundant, longer chains on this medium. The chains are easier to observe because there is less moisture on the surface of the agar and fewer droplets of moisture in the aerial mycelium.
Soil Extract Agar
Soil is a natural medium for many organisms as they provide a perennial source of organic matter and other sources of carbon, nitrogen, minerals, and vitamins required for their growth. Soil Extract Agar is a medium used for isolating soil microorganisms like bacteria, actinomycetes, fungi, algae, and protozoa. Soil extract provides all the essential nutrients required for the growth of such microorganisms. Glucose serves as a readily metabolizable carbon source whereas dipotassium phosphate buffers the medium. Soil agar promotes rapid chlamydospore formation in a number of Fusarium species. Large inoculum with actively growing fusarium inoculates produces chlamydospores within 3-4 days but secondary inoculates produce chlamydospores in 30 days.
Pathogenesis of Fusarium
Fusarium species cause diseases in plants, animals, and human hosts, but most commonly in plants. These pathogenesis have been linked to the toxigenicity of the species associated with the production of mycotoxins such as trichothecenes (types A and B), fusaric acid, and fumonisins. Trauma is the major predisposing factor for the development of cutaneous infections whereas neutropenic and transplant hosts are for disseminated opportunistic infections. Fusarium species cause superficial, locally invasive, and diffuse infections in humans. Localized infection includes septic arthritis, Keratitis, osteomyelitis, otitis media, cystitis, onychomycosis, cutaneous infections particularly burn wounds, mycetoma, sinusitis, pulmonary infections, endocarditis, peritonitis, central venous catheter infections, and brain abscess. Invasive infections are a result of surgery and oral antifungal therapy. Disseminated infection occurs when two or more noncontiguous sites are involved. Fungemia and outbreaks of nosocomial fusariosis have also been reported. The infections are opportunistic and they are majorly caused by F. solani complex , F. oxysporum, F. verticillioides, and F. proliferatum, F. moniliforme and F. fujikuroi species complex.
They cause opportunistic infections in immunocompromised patients. The elderly and diabetics with prevalent meningospondylodiscitis are opportunistically infected by F. oxysporum. F. sacchari, F. anthophilum, F. chlamydosporum, and F. dimerum. A perinephric abscess caused by F. chlamydosporum is common in children who have been reported before. Corneal infections (endophthalmitis) caused by F. oxysporum and F. solani also occur because of the adherence of the fungi to the corneal membrane causing eye damage. Some Fusarium species, such as F. dimerum, are associated with keratomycosis, particularly in bad hygiene conditions. Mycotoxicosis caused by Fusarium species is common in the ingestion of the mycotoxins produced by the fungi.
Clinical manifestation of fusariosis in immunocompromised hosts
- Endophthalmitis
- Sinusitis
- Pneumonia
- Skin Involvement
- Fungemia
- Disseminated Infection
Laboratory Diagnosis of Fusarium
Specimen: It depends on the nature of the infection site e.g. in case of fungemia, blood, in the diagnosis of keratitis corneal scrapings (most frequent) or tissue biopsy and skin lesions (either cellulitis or metastatic lesions), and also blood for cultures for mold.
KOH mount: Presence of fungal elements
Fungal culture: To obtain growth of fungi. Growth of Fusarium oxysporum on SDA and its LPCB preparation as shown below video-
LPCB preparation: Observation of fungal structures from culture.
Serological test: β-d-Glucan Testing Is Important for Diagnosis of Invasive Fungal Infections but cannot distinguish Fusarium from other fungal infections (Candida, Aspergillus, Trichosporon, and others) which are also detected by the assay.
- Histopathological examination: It is also a helpful tool for confirmatory diagnosis of fusariosis. In tissue, the hyphae are similar to those of Aspergillus species, with hyaline and septate filaments that typically dichotomize in acute and right angles. Although, adventitious sporulation may be present in the tissue, and the finding of hyphae and yeast-like structures together is highly suggestive of fusariosis in the high-risk population. In the absence of fungal growth, distinguishing fusariosis from other hyalohyphomycoses (a group of opportunistic mycotic infections caused by nondematiaceous molds) may be difficult and requires the use of another technique in situ hybridization in paraffin-embedded tissue specimens.
Molecular test: PCR-based method, using sequencing identification as a gold standard but why this, it verifies as identification of Fusarium species is often difficult due to the variability between isolates and because not all features required are always well developed (e.g. the absence of macroconidia in some isolates after subculture). It is possible to identify the genus Fusarium by several methods. On culturing, hyaline, banana-shaped, and multicellular macroconidia are very common; however, to identify them at the species level is not easy. Therefore, molecular methods are needed. Some of the most commonly used molecular methods are genus-specific PCR, 28 s rRNA gene sequencing, sequence-based PCR, multiplex tandem PCR, and automated repetitive sequence-based PCR.
Treatment
Useful antifungal drugs are-
- Itraconazole
- Voriconazole
- Amphotericin B and
- Posaconazole.
Prevention and Control and Fusarium species
General
- Prognosis in fusariosis is poor and the limited susceptibility of Fusarium spp. to antifungal agents and therefore prevention of infection remains the cornerstone of management. Reducing immunosuppression should be attempted in patients with an earlier history of Fusarium infection and can be achieved by a reduction in or discontinuation of immunosuppressive agents, shortening the duration of neutropenia.
- Skin evaluation is also mandatory before giving immunosuppressive therapy because the skin may be the source for disseminated and frequently life-threatening Fusarium infections.
- Any area of tissue breakdown should be identified and suspicious skin lesions cultured and biopsied. Local debridement should be performed and topical antifungal agents e.g. Natamycin, amphotericin B considered if Fusarium species are identified.
Antifungal Agent Prophylaxis
There are no recommendations for antifungal prophylaxis against Fusarium species either as primary prophylaxis or as secondary prophylaxis (patients with prior fusariosis who will be exposed to periods of prolonged neutropenia or will undergo an allogeneic HSCT). However, the use of an antifungal agent should be considered for secondary prophylaxis, and the choice should be based on the Fusarium species causing infection and/or the results of in vitro antifungal susceptibility testing (AFST) if available.
Infection Control
Since the airways are the principal portal of entry for Fusarium species, the placement of patients at high risk (prolonged and profound neutropenia and allogeneic HSCT recipients) in rooms with HEPA filter and positive pressure may decrease the risk of nosocomial acquisition of fusariosis. In addition, since the water may be a source of Fusarium species in the hospital, every effort should be made to prevent patient exposure (e.g., by avoiding contact with reservoirs of Fusarium spp., such as tap water, and/or cleaning showers prior to use by high-risk patients during periods at risk. For human and animal infections can be treated with intravenous administration of itraconazole, oral amphotericin B.
Others attempts
- Fungicides have very minimal effects with likely resistance development on most of the Fusarium species groups.
- Resistant cultivars(a plant variety that has been produced in cultivation by selective breeding) can be used to control Fusarium Head Blight by controlling the plant lines that allow the release of mycotoxins. This reduces fungal growth and lowers mycotoxin contamination.
Key Notes on Fusarium species
- Fusarium differs from comparatively matching some fungi like Acremonium, Lecythophora, and Phialemonium by having macroconidia. It differs from Cylindrocarpon by having macroconidia with foot cells and pointed distal ends.
- Fusarium oxysporum: In contrast to Fusarium solani the phialides are short and mostly non-septate. Fuasrium solani growth on PDA and its LPCB mount under the microscope as shown in this video-
- Macroscopic and microscopic features, such as the color of the colony, length and shape of the macroconidia, the number, shape, and arrangement of microconidia, and presence or absence of chlamydospores are key features for the differentiation of Fusarium species.
- Molecular methods, such as 28S rRNA gene sequencing, may be used for the rapid identification of Fusarium strains to species level.
- Plant diseases are Fusarium head blight (FHB) caused by F. graminearum, which contributed to the loss of starch and proteins in cereals, Footrot (FR) and root rot (RR), crown rot (CR), Fusarium wilts a destructive disease in bananas caused by F. oxysporum whereas Pokkah Boeing on sugarcane and banana disease of rice.
Phialophora species: General Characteristics, Pathogenesis, Clinical Findings, Laboratory Diagnosis, Treatment, Prevention, and Control
Taxonomic classification of Phialophora
( Medlar in 1915)
Kingdom: Fungi
Phylum: Ascomycota
Class: Euascomycetes
Order: Chaetothyriales
Family: Herpotrichiellaceae
Genus: Phialophora
Species: P. verrucosa, P. americana, P. bubakii, P. europaea, P. parasitica, P. reptans, P. repens, P. richardsiae, and P. europaea.
Note: Morphological features, such as the shape of the collarettes, organization of the phialides, the existence of chlamydospores, and biochemical features, such as the assimilation of melibiose help in the differentiation of the species from each other
Habitats of Phialophora
Phialophora is a dematiaceous fungus that inhabits the soil, plants, and decaying food; and is widely distributed in nature. Phialophora spp. are the causative agents of some human infections like chromoblastomycosis, mycetoma, and phaeohyphomycosis.
General Characteristics of Phialophora
Phialophora contains more than 40 species, among them medically important are P. verrucosa, P. americana, P. bubakii, P. europaea and P. reptans. Both P. verrucosa and P. americana produce their conidia from phialides with conspicuous darkened collarettes, however, sequencing has demonstrated a close relatedness, suggesting that these species may be synonymous. P. verrucosa is primarily an agent of chromoblastomycosis since it is the second most common cause of chromoblastomycosis worldwide (after Fusarium pedrosoi), mycetoma, and phaeohyphomycosis although other reported infections include endocarditis, keratitis, and osteomyelitis.
Risk group: It comes in a risk group (RG)-2 organism.
Morphological Description of Phialophora verrucosa
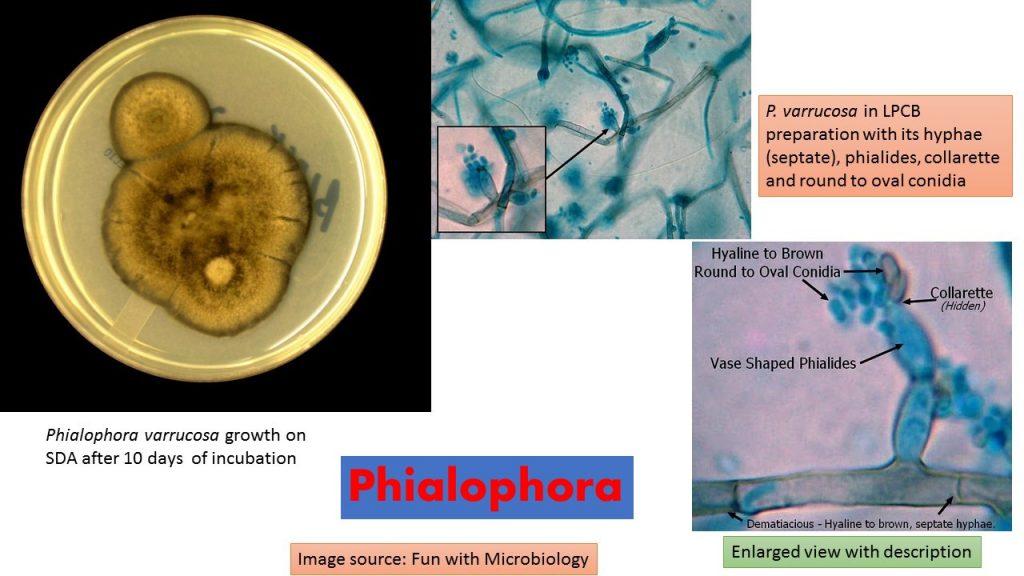
Colonies on Sabouraud dextrose agar (SDA) are slow-growing, initially dome-shaped, later becoming flat, suede-like, and olivaceous to black in color.
Phialides are flask-shaped or elliptical with distinctive funnel-shaped, darkly pigmented collarettes.
Conidia are ellipsoidal, smooth-walled, hyaline, mostly 3.0-5.0 x 1.5-3.0 μm, and aggregate in slimy heads at the apices of the phialide.
Pathogenicity of Phialophora
Phialophora species are among the causes of chromoblastomycosis and phaeohyphomycosis. P. verrucosa is the principal causative agent of chromoblastomycosis in tropical and subtropical areas, particularly in Japan and South America. The clinical forms of phaeohyphomycosis may be diverse, including cutaneous infections, subcutaneous cysts, keratitis, endocarditis, arthritis, osteomyelitis, cerebral infection, fatal hemorrhage, and disseminated infection. P. europaea has been isolated from cutaneous and nail infections in North-western Europe.
Clinical Findings
It depends on the site of infection. e.g. in the case of chromoblastomycosis. It involves the foot or leg, but other exposed body parts may be infected, especially where the skin is broken. Initially small, itchy, enlarging papules may resemble dermatophytosis (ringworm). These papules extend to form dull red or violaceous, sharply demarcated patches with indurated bases. The later untreated case may cause Lymphatics obstruction and itching may persist, and secondary bacterial superinfections may develop, causing ulcerations and occasionally septicemia. Phaeohyphomycosis may show the following clinical manifestations-invasive sinusitis, subcutaneous nodules or abscesses, keratitis, lung masses, osteomyelitis, endocarditis, mycotic arthritis, brain abscess, and disseminated infection.
Laboratory Diagnosis of Phialophora
Specimen: It depends on the nature of the infection site e.g. in the case of chromoblastomycosis skin scrapping or tissue biopsy whereas in keratitis corneal scrapings (most frequent) or tissue biopsy and skin lesions (either cellulitis or metastatic lesions) and also nails clipping in case of nail infection.
KOH mount: Presence of fungal elements
Fungal culture: To obtain growth of fungi. Exhibits slow to moderate growth, usually maturing in about 7 to 12 Days. Colonies are woolly to velvety, dark grey, brown, or olivaceous black on the surface and reverse.
LPCB preparation: Observation of fungal structures from culture. Dematiacious (melanin pigment) – Hyaline to brown, septate hyphae. Phialides are pale brown to brown, bottle or vase, or shaped with a darker collarette at the apical end. Phaialides are located laterally or terminally on the hyphae.
Conidia are unicellular, smooth and thin-walled, hyaline to brown and round or ovoid (1-3 X 2-4 µm) which accumulate at the apex of the collarette giving the appearance of a vase of flowers.
Histopathological examination: The organisms appear as dark round cells, 5 – 12 µm in diameter. Similar to the other fungi causing chromoblastomycosis, spherical or polyhedral, dark brown, thick-walled sclerotic bodies (muriform cells) are visualized in tissues infected with Phialophora species. The absence of muriform cells has been reported in cases that are immunosuppressed or debilitated. Phaeoid hyphae may also be observed.
Molecular test: Internal Transcribed Spacer (ITS) sequencing recommended (de Hoog et al. 1999).
Treatment of Phialophora
Useful antifungal drugs are-
- Itraconazole
- Voriconazole
- Amphotericin B and
- Posaconazole.
Key Notes on Phialophora
- Some human pathogens with phialidic conidiogenesis previously assigned to Phialophora have been moved to other genera, namely, Phaeoacremonium and Pleurostomophora.
- Chromoblastomycosis is a chronic fungal infection of the skin and the subcutaneous tissue caused by traumatic inoculation of a specific group of dematiaceous fungi (usually Fonsecaea pedrosoi, Phialophora verrucosa, Cladosporium carrionii, or Fonsecaea compacta) through the skin.
- Phaeohyphomycosis is a chronic infectious condition caused by dematiaceous fungi which usually involve the skin and subcutaneous tissue.
- ITS rRNA Gene-Based Phylogenetic Reconstruction Using Algorithms with Local and Global Sequence Alignment for Black Yeasts and Their Relatives
- Organisms that are similar to Phialophora are Exophiala, Fusarium, Lecythophora, Phaeoacremonium, Phialemonium, and Wangiella.
- Some differentiating features are as follows:
- Phialophora differs from Exophiala by having phialides and from Wangiella by having phialides with collarettes.
- P. richardside : Phialides with saucer-shaped or flared collarettes.
- P. verrucosa: Collarettes vase-shaped, darkly pigmented; phialides flask-shaped.
- Lecythophora hoffmannii: Colonies first yeast-like, flat, cream-colored, turning pink to salmon (old cultures turning black in the center); phialides not separated from hyphae by a septum; conidia one-celled, often curved.
- Phialemonium species: Colonies yeast-like (some), flat, fast-growing, white, cream-colored, light gray, some with the green color or light vinaceous color; tapering phialides without a basal septum, branching phialides; conidia one-celled, obovate or curved in shape.
- P. parasitica: Phialides cylindrical to obclavate, elongate, hyaline to pale brown
- P. repens: Phialides cylindrical to lageniform, short, hyaline to pale brown.
Prevention and control of Phialophora
- Store food properly to save it from contamination.
- Broken skin parts are prone to infection and thus follow safety guidelines.
- Maintain personal hygiene.
- No vaccine is available.
- To control the infection, some anti-fungal drugs are available.
Trichophyton mentagrophytes: Introduction, Morphology, Pathogenicity, Laboratory Diagnosis, and Key Notes
Trichophyton mentagrophytes
Trichophyton mentagrophytes colony characteristics on SDA are white to tan, cottony or powdery, pigment variables as shown above picture. Superficial fungal infections are a major global public health problem that affects 20–25% of the population worldwide. Among these diseases, dermatophytosis, or tinea, is one of the most frequent fungal infections. This infection is caused by dermatophyte species that belong to the Trichophyton, Microsporum, or Epidermophyton genera. T. mentagrophytes are filamentous fungi, digest keratin, and do not invade living tissues. They are incapable of penetrating subcutaneous tissue.
Morphology of Trichophyton mentagrophytes
This fungus is characterized morphologically based on the development of macro and microconidia with smooth walls. The colony is white to tan, cottony or powdery, pigment variables. The macroconidia originate laterally in the hyphae or in short pedicles of thin or thick walls and are club-shaped or fusiform, with a size that varies from 4-8 to 8-50 μm. The microconidia are abundant, spherical, pyriform, or irregularly shaped, with sizes varying from 2-3 to 2-4 μm. The most consistent feature of T. mentagrophytes is the production of globose micro-aleuriospores arranged in groups (like a bunch of grapes).
Pathogenicity of Trichophyton mentagrophytes
Dermatophytes are a group of fungi that are closely related to each other and have the enzyme keratinase; thus, they can cause infections in the skin, hair, and nails in both humans and animals. Among the dermatophytes, T. mentagrophytes stands out as the second most common causative agent of dermatophytosis after Trichophyton rubrum.
Mode of infection:- Acquired by direct contact with soil, animals, or humans infected with fungal spores.
Predisposing factors – Moist humid skin and tight-fitting underclothing.
Skin: grow in a centrifugal pattern in the stratum corneum annular or ring-shaped pruritic scaly skin lesions with central clearing and raised edges.
Nails: invade nails through lateral or superficial nail plates and then spread throughout the nails.
Hair shaft: Invade the hair shaft or may be found surrounding it. Hairs become brittle and areas of alopecia may appear. Males more commonly infected as progesterone is inhibitory to dermatophyte growth.
Incubation period:- 1 to 2 weeks.
Anthropophilic dermatophytes:- commonest, cause relatively mild and chronic lesions but respond poorly to treatment.
Geophilic and zoophilic species:- less adapted to humans produce a more acute inflammatory response and severe infections; but they tend to resolve more quickly.
Clinical Types
Tinea capitis: Infection of the scalp (various types)
Kerion:- Painful inflammatory reaction producing boggy lesions on scalp Favus:- Cup like crust (scutula) forms around the infected hair follicle with minimal hair shaft involvement
Ectothrix:- Arthrospore formation occurs on the surface of the hair shaft
Endothrix:- Arthrospore formation occurs within the hair completely filling the hair shaft.
Tinea corporis: Infection of the non-hairy skin of the body e.g. trunk and limbs
Tinea pedis: Infect first the webs between the toes, then spread to the sole in a “moccasin” pattern. It is also called Athlete’s foot.
Tinea cruris ( Jock itch): Infection of the groin area
Tinea barbae: Infection of the beard and mustache area of the face
Tinea facie: Infection of the non-bearded area of the face
Tinea imbricate: Concentric lesions of the skin
Tinea unguium( nail plate infection): Infection of nail beds
Tinea manuum: Infection of the palmar aspect of hands
Laboratory Diagnosis of Trichophyton mentagrophytes
Specimens: It depends on the site of infection. e.g. Skin scraping in case of a skin infection, hair plucks for hair infection, and nail clipping from the active margin of the lesions of the nail.
Transportation of specimen:-In moisture-free paper but when scrapings are to be sent through the post, they should be folded in thick black paper.
KOH Mount: branching septate hyaline mycelia, which frequently show arthrospore production. Hair- arthroconidia on the surface of the shaft (ectothrix) or within the shaft (endothrix). Septate hyaline hyphae and arthrospores of dermatophytes
Culture: Sabouraud dextrose agar (SDA) containing cycloheximide and incubated at 25°C, 30°C, and 37°C for 4 weeks. Potato dextrose agar – better sporulation (useful for the production of pigment). Colonies appear in 10 days to 3 weeks, depending on the organism strain. Dermatophytes test Medium (DTM)is used for presumptive identification of dermatophytes from fungal or bacterial contaminants found prevalent in cutaneous lesions. Incubation at 25°C.
Physiological characteristics
Urea hydrolyzation test: Positive
and hair perforation test: Positive
LPCB Preparation: LPCB stain stands for lactophenol cotton blue and it is a combination of fixative, staining, and clearing agent. LPCB uses both as a mounting fluid and a stain. This is used for staining and microscopic identification of fungi. Its contents functions are as follows- Lactic acid: It helps in preserving the morphology of the fungal elements. Phenol: It acts as a disinfectant. Cotton blue: It stains the fungal elements as well as intestinal parasitic (cyst, ova, and oocyst) and non-parasitic structures (vegetable cells, mucus, muscle fibers, and other artifacts). Glycerol: It is a hygroscopic agent that prevents drying. Tease the colony for LPCB mount which helps to demonstrate the hyphae and spore (conidia). Conidia are of two types, microconidia -small unicellular while macroconidia are multicellular and septate. Special hyphae may have spiral hyphae, racquet hyphae, and favic chandeliers.
Molecular Identification: Internal transcribed spacer (ITS) region of the nuclear ribosomal DNA (rDNA) sequencing may be used to identify clinical species. The sequencing of a fragment from the region ITS1-5.8S-ITS2 of T. mentagrophytes complex was performed in the study of ‘ Molecular identification of isolates of the Trichophyton mentagrophytes complex’ in Mexico.
Skin test:-It detects hypersensitivity to trichophytin.
Treatment of dermatophytosis
Oral terbinafine or itraconazole is the drug of choice for the treatment of dermatophytosis.
Duration:- depends on the affected site (1 – 2 weeks for skin lesions, 6 weeks for hair infection, 3 months for onychomycosis)
They can be given as pulse therapy.
Alternative:- Oral griseofulvin and ketoconazole
Topical lotion:- Whitfield ointment or tolnaftate
Key Notes on Dermatophytes
- The taxonomy of T. mentagrophytes is complex due to the changes it has undergone in recent years. Until 2017, T. mentagrophytes-series included seven species: T. tonsurans, T. mentagrophytes, T. interdigitale, T, equinum, T. quinckeanum, T. schoenleinii, and T. simii characterized through ecological data, morphological characteristics, mating type studies, and molecular analysis.
- However, nowadays, only five species are considered—T. mentagrophytes, T. interdigitale, T. erinacei, T quinckeanum, and T. benhamie—as well as nine different genotypes of T. mentagrophytes / T. interdigitale associated with the geographical origin and the source of infection
- Conventionally, T. mentagrophytes is identified based on their macro and microscopic features, and sometimes, for their physiological characteristics i.e. hair perforation and urease activity.
- Woods Lamps Examination:- Positive for various Microsporum species and Trichophyton schoenleinii. Fluorescence is due to the presence of pteridine pigment in the cell wall.
- Key features of Trichophyton mentagrophytes are hyaline, septate, and branched hyphae as well as abundant spherical or semi-spherical microconidia that resemble clusters of grapes, spherical chlamyconidia, spiral hyphae, macroconidia, and nodular bodies.
- Trichophyton mentagrophytes and Microsporum canis are hair perforation test positive in which fungi pierce hair-producing wedge-shaped perforations.
Related Videos
#Ringworm and its causative agent dermatophyte under the microscope as shown below-
#Dermatophytes in KOH Preparation-
#Dermatophyte causing disease and its lab diagnosis |Trichophyton | Microsporum |Epidermophyton:
Dermatophytes are fungi that require keratin for growth. These fungi can cause superficial infections of the hair, skin, and nails consisting of three genus- Trichophyton, Microsporum, and Epidermophyton -spread by direct contact. Laboratory diagnosis on the following features -Site of infection, Colonial morphology, Presence of spores on LPCB tease mount- Microconidia and Macroconidia-
#Trichophyton mentagrophyte Isolated:
features- Helical pattern on LPCB Mount seen
Urease test-Positive
Hair perforation test-Positive
#Trichophyton rubrum– growth on dermatophyte test medium (DTM)
and its colonial morphology and LPCB tease mount under microscopy as shown in the video.
Microsporum: General Characteristics, Pathogenesis, Clinical Findings, Laboratory Diagnosis, Epidemiology, Prevention, and Control
Introduction of Microsporum
Microsporum is a genera dermatophytes that cause dermatophytosis, or cutaneous infections of the hair and skin. They are ascomycetous molds that produce macroconidia spores that are unique to this group of dermatophytes, distinguishing them from Trichophyton and Epidermophyton. Microsporum spp. are keratolytic, meaning they contain keratinase, an enzyme that digests skin and hair particles, causing cutaneous diseases. Since it cannot develop in temperatures above 37°C, it is restricted to nonviable skin tissues as a dermatophyte. It is a zoonotic fungus since it is extremely transmissible from animals to humans.
Risk Group Classification
Risk Group 2 pathogens.
Clasification of Microsporum
(Gruby 1843)
Kingdom: Fungi
Division: Ascomycota
Class: Eurotiomycetes
Order: Onygenales
Family: Arthrodermataceae
Genus: Microsporum
Other species: Microsporum fulvum, Microsporum amazonicum, Microsporum boullardii, Microsporum cookei, Microsporum distortum, Microsporum duboisii, Microsporum ferrugineum, Microsporum fulvum.
Habitat of Microsporum
Microsporum live in the soil and are found all over the world. They grow at a room temperature of 25-27 degrees Celsius, on keratin surfaces with low temperatures. They are keratotic, so they tend to live in keratin-rich environments including hair and skin.
Susceptibility to Disinfectants
Microsporum are susceptible to phenolic compounds, formaldehyde, glutaraldehyde, iodophors, and sodium hypochlorite (1%).
Physical Inactivation
The infectious substance can be inactivated by UV, gamma, and microwave (aerosol) radiation; moist heat (121°C for at least 20 minutes; and dry heat (165-170°C for 2 hours).
Morphology
Asexual reproduction occurs in Microsporum, which produces macroconidia and microconidia. Macroconidia are asexual spores of large size. They are hyaline, multiseptate, with a range of shapes ranging from spindle-like to obovate. The macroconidia have a thin or thick echinulate to the verrucose cell wall and are 7–20 µm by 30–160 µm in height. These fungi can be distinguished from other dermatophytes by their distinct form, which is dense and rough. Microconidia are smaller than macroconidia and are therefore asexual Microsporum spores. Microconidia are single-celled hyaline organisms. Microconidia have a smooth cell wall and are pyriform to clavate in shape.
Microconidia are 2.5–3.5 µm long and 4–7 µm wide.
Culture Characteristics
At 25°C, they develop quickly on Sabouraud dextrose agar (SDA). Microsporum spp. can produce a perfect shape, which is a fungus that can complete meiosis. In rice grain medium with pigmented peripheries, they grow well. The basic media for the growth and identification of Microsporum species is Trichophyton agar.
Common species of Microsporum
Microsporum audouinii
They are anthropophilic fungi that cause non-inflammatory scalp and skin infections in children. It is a natural cause of tinea capitis, entering hair shafts and inducing an ectothrix infection. The ectothrix fluoresces a bright yellow under Wood’s ultra-violet light. They form spread flat colonies that vary in color from whitish-grey to tanned white on simple media. They have a furry look about them. They produce macroconidia and very rarely microconidia. The macroconidia are long and smooth, with a spindle shape that is irregular. Microconidia are pyriform to clavate in shape if they form. They also produce pectinate (comb-like) and racquet hyphae (segmented series of hyphae with swollen tips).
Microsporum canis
This is a zoophilic dermatophyte that is spread worldwide and is transmitted from dogs and cats to humans. Ringworms are commonly caused by it, particularly in children. It causes an ectothrix infection, which can be detected using Wood’s ultra-violet light, which fluoresces as a bright greenish-yellow light. They grow well in rice grain agar, producing white aerial mycelium as well as a yellow pigment. They form white to cream-colored spread flat colonies with a cottony surface in basic media. The colonies also have a brownish-yellow-edged pigmented surface. They create spindle-shaped macroconidia with 5-15 cells, a verrucose thick wall, and a terminal end. They may also produce microconidia that are pyriform to clavate and produce fewer microconidia. Microsporum canis var. distortum, which causes infection in cats and dogs, and Microsporum canis var. equinum, which causes ringworms in horses, are two other dysgonic strains of Microsporum canis.
Microsporum ferrugineum
It’s an anthropophilic fungus that causes human tinea capitis epidemics in children. It infects people in the same way that Microsporum audouinii does. It infects the hair shafts, causing an ectothrix infection that is visible as greenish-yellow fluorescence in the Wood’s ultra-violet light. Asia, Russia, Africa, and Eastern Europe are all familiar with it. Colonies form slowly in basic mycological media (SDA, PDA) and have a waxy glabrous, convoluted thallus with a creamy to buff-colored surface. Surface pigmentation ranges from yellow to creamy to dark red. The colonies are pleomorphic and downy. They also have irregular branching hyphae that look like bamboo hyphae and carry chlamydospores. The bamboo-branched hyphae is a specific and distinct characteristic of Microsporum ferrugineum as shown above the image.
Mode of Infection
Direct or indirect contact with desquamated epithelium-contaminated skin or scalp lesions of infected individuals, animals, or fomites. In people with weakened cell-mediated immunity, the infection can spread via broken skin.
Pathogenesis of Microsporum
Microsporum spp. virulence is attributed to their ability to colonize keratin-containing materials like skin, hair, and nails, indicating that it is a fungal keratinase. Keratinase is an enzyme produced by fungi that degrade and breaks down keratin materials. Because the body’s defense mechanisms prevent the fungi from invading the circulatory system, the immune response elicited by the host is a localized inflammatory response. When fungi colonize the mucous membrane, they release the elastase enzyme, which activates the immune system. Microsporum, like other dermatophytes, causes cutaneous infections on hair, skin, and nails. As a result, the fungi are known to cause a variety of cutaneous infections (dermatophytosis), such as Tinea capitis and Tinea corporis.
Incubation Period
The incubation period is from several days to a few weeks, depending on the species and the host.
Tinea capitis
This is a fungus that infects the hairs on the scalp and is also known as ringworms or herpes tonsurans. Epidermophyton and Trichophyton spp. are also responsible. The fungi infiltrate the hair shafts by penetrating the outer root sheath of the hair follicles. It may result in either an inflammatory or non-inflammatory infection. Inflammatory tinea capitis is marked by painful pus-filled nodules and scarring alopecia, in which the affected area loses hair and leaves a scar. Eyelashes and brows may also be affected. It is most common in children aged 3 to 14, but it can affect people of any age, particularly those who are immunocompromised, such as diabetics or cancer patients. Tinea capitis is a Microsporum spp. specific ectothrix infection in which the fungi attack the outer sheath root. Subacute and chronic dermatitis with or without follicular inflammation and destruction characterize it. It’s possible that suppurative folliculitis is present. Hyperkeratosis is a type of hyperkeratosis. Parakeratosis is a type of keratosis that affects (a mode of keratinization characterized by the retention of nuclei in the stratum corneum).
Tinea corporis
It is a pink-reddish round-shaped patch with plaques and a raised scaly border that extends on the periphery and clears at the center, also known as ringworms. Small pus-filled papules have appeared on the periphery. It causes itching, scaling, redness, and rashes on the arms and legs, and it can be dry and flaky.
Spongiosis
An inflammatory infiltrates in the perivascular space, black dot tinea capitis, which infects the hair, is one of the clinical symptoms. Kerion is characterized by inflammation that leads to alopecia scarring (a condition that causes hair to fall out in small patches, which can be unnoticeable). Favus is a bog inflammatory condition characterized by deep-seated oozing nodules, abscesses, crusting, or scutula. The distinctive lesion of favus, a yellow saucer-shaped crust containing a mass of hyphae and spores).
Laboratory Diagnosis of Microsporum
Specimen: It depends on the site of infection. e.g. in skin infection, skin scrapings, nail infection-nail scrapings whereas in hair infection- hair scraping, pus, tissue biopsies.
Differential diagnosis: It is necessary to clear from other resembling conditions like a bacterial abscess, psoriasis, eczema, syphilis, lupus erythematosus.
Direct Microscopy
Potassium hydroxide (KOH) Wet Mount: May show fungal elements
Culture Characteristics
Potato Dextrose Agar (PDA): M. audouinii produces pinkish-brown or salmon-colored fluffy colonies while M. canis produces bright yellow colonies.
Trichophyton Agar
M. audouinii produces flat, white, suede-like to downy, with yellow-brown reverse colonies with a furry texture whereas M.canis forms flat, white, suede-like to downy, with a yellow to pale yellow-brown reverse colonies.
Rice Grain Agar
M. canis produces white aerial mycelium with the production of yellow pigment
Histological Identification
The periodic acid-Schiff (PAS) stain is a special stain that will help in identifying dermatophytic fungi.
LPCB Tease mount
It is used for the observation of fungal structures like hyphae and spores or conidia. Conidia is of two types i.e. Microconidia:- small unicellular
Macroconidia:- Multicellular, septate
Special hyphae:- hyphae such as spiral hyphae, racquet hyphae, and favic chandeliers
Biochemical test
Urease test:- Microsporum species are urease negative
Other methods of diagnosis
Hair perforation test: – fungi pierce hair-producing wedge-shaped perforations.
Positive: – Trichophyton mentagrophytes and Microsporum canis.
Molecular methods: PCR assay
Woods Lamps Examination: – Positive for various Microsporum species and Trichophyton schoenleinii. Fluorescence is due to the presence of pteridine pigment in the cell wall. Fluorescence is seen under wood’s lamp-
Microorganism Fluorescence Color
- Microsporum audouinii : Bright – green
- M. canis: Bright–green
- M. ferrugineum: Blue – green
- M. distortum: Blue-green
- M.gypseum: Dull – yellow
- Trichophyton schoenleinii: Dull – green
- Malassezia furfur: Golden – yellow
Treatment
- Antifungals in cream, lotion, or gel, such as imidazole, ciclopirox, naftifine, or terbinafine, for mild-moderate lesions caused by tinea corporis and tinea capitis.
- Oral itraconazole can be used to treat severe infections for an extended period of time.
- Antifungal shampoos are used for both treatment and prevention of fungal infection spread. Tinea capitis and tinea corporis can both be prevented with antifungal creams.
Prevention and Control
- Handwashing after handling and exposure to animals, soil, and plants should be done in a sanitary manner.
- Touching lesions on the skin and scalp of infected individuals should be avoided.
- On the affected areas, loose-fitting clothing is recommended.
- When participating in activities that require close human contact with skin, it is important to maintain good hygiene.
Epidemiology
This pathogen is found all over the world, and infections are fairly common. They are the most common pathogens in northern Europe and North America. Southern Europe and the Arabic countries have a higher prevalence of zoophilic dermatophytes.
Keynotes
- The absence of microconidia and macroconidia, having bamboo hyphae( irregular branching hyphae with prominent cross-walls) and the presence of chlamydospores are the diagnostic features of Microsporum ferrugineum.
- Microsporum persicolor properties-
Colony characteristics
White to pinkish in color
Reverse pigment: orange
Microconidia: thin-walled and cigar-shaped
-Abundant, spherical to pyriform
4-7 celled microconidia may also be found but rarely.
- Pectinate body is found in M. audouinii.
- The nodular organ is found in M. canis.
Epidermophyton: General Characteristics, Pathogenesis, Clinical Findings, Laboratory Diagnosis, Treatment, Prevention, and Control
Epidermophyton General features
Epdidermophyton is one of the members of dermatophytes. Dermatophytoses (tinea or ringworm): It is the most common superficial mycosis affecting skin, hair, and nails. These are closely related keratinophilic fungi ( three genera, Trichophyton, Microsporum, and Epidermophyton), which are capable to invade keratinized tissues of the skin and its appendages and are correctively known as Dermatophytes. Characteristics of dermatophytes are as follows:
- Filamentous fungi, digest keratin.
- Do not invade living tissues.
- They are incapable of penetrating subcutaneous tissue.
Morphological classification
Dermatophytes are hyaline septate molds with more than a hundred species described. These are divided into three main anamorphic genera depending on their morphological characteristics, according to shape and site infections.
Trichophyton:- pencil-shaped, infect skin, hair, nails e.g. T. rubrum T. mentagrophytes, T. tonsurans, T. violaceum.
Microsporum:- spindle shaped, infect skin and hair e.g. M. audouinii, M. canis, M. equinum, M. gypseum
Epidermophyton:- Club shaped, infect skin and nails e.g. E. floccosum and E. stockdaleae
Ecological classification
Depending on the usual habitat (Humans, animals, and soil).
- Anthrophilic :- infected Humans. e.g. T. rubrum ,T. mentagrophytes, T. schoenleinii, T. tonsurans, T. violaceum , M. audouinii and E. floccosum
- Zoophilic :- infect animals as well as birds. e.g. T.equinum, T. verrucosum, M. canis and M. equinum
- Geophilic :- frequently isolated from soil. e.g. T. ajelloi , M. gypseum , E. stockdaleae
Epidermophyton floccosum
Epidermophyton floccosum is an anthropophilic dermatophyte with a cosmopolitan distribution that often causes tinea pedis, tinea cruris, tinea corporis, and onychomycosis ( nail infection). E. floccosum is not known to invade hair in vivo and no specific growth requirements have been reported.
Risk Group
RG-2 organism
Morphology of Epidermophyton floccosum
Colonies of E. floccosum are usually slow-growing, greenish-brown or khaki-colored with a suede-like surface, raised and folded in the center, with a flat periphery and submerged fringe of growth but above image lacking such properties due to being young culture ( 9 days of incubation at 25°C). Older cultures may develop white pleomorphic tufts of mycelium. A deep yellowish-brown reverse pigment is usually present. Microscopic morphology shows characteristic smooth, thin-walled macroconidia which are often produced in clusters growing directly from the hyphae (specific feature- having racquet hyphae as shown above image). Numerous chlamydospores are formed in older cultures while microconidia are not formed.
Pathogenesis of Epidermophyton floccosum
Epidermophyton is a keratinophilic filamentous fungus. The ability to invade keratinized tissues and the possession of several enzymes, such as acid proteinases, elastase, keratinases, and other proteinases are the major virulence factors of these fungi.
Clinical Manifestations of Epidermophyton
Tinea pedis or Athlete’s foot: It is the ringworm, fungal infection of feet involving interdigital webs and sole. The most common clinical finding is an intertriginous form associated with maceration, scaling, fissuring, and erythema which presents with itching and burning sensation. The most common causative agents are E. floccosum, T. rubrum and T. mentagrophytes. It is common among athletes and office workers and due to the constant wearing of shoes with synthetic nylon socks that do not absorb sweat.
Tinea cruris or Jock itch or Dhobie’s itch: It is ringworm of the inguinal area involving the groin, perianal, perineal areas often involving the upper thigh. Common species of dermatophytes involved are T. rubrum, T. mentagrophytes and E. floccosum. It is mainly seen among students as they mostly wear synthetic tight under-garments in which sweat does not get absorbed and long-standing moisture predisposes to fungal infection.
Tinea corporis: Ringworm of glabrous skin. The lesions are well marginated with raised erythematous borders. The annular, scaly patches may coalesce to form a large area of chronic infection. The common causative agents of dermatophytes are T. rubrum, T. mentagrophytes, T. tonsurans but also the involvement of E. floccosum. It is is observed to be predominant among people with a previous family history of the disease and it may be transmitted by direct contact with other infected individuals. Tinea capitis is the second most important clinical type seen among people with a previous family history of the disease. It is because these diseases may be transmitted through fomites such as comb, hairbrushes, bedding, pillows, clothes, towels or furniture, etc. In addition, tinea corporis can be attributed to poor personal hygiene and heavy manual work.
Tinea unguium or onychomycosis: It is the ringworm infection of the nail plate. Distal subungual infection is the commonest pattern and involves the nail bed and underside of the nail in the distal portion. The nail plate is brittle, friable, thickened, and may crack because of piling up of subungual debris. The color of the nail becomes often brown or black. The commonest species responsible for causing onychomycosis are tinea unguium are T. rubrum, T. mentagrophytes, and E. floccosum. It is common among housewives and servant maids due to the practice of cleaning the cowshed bare-handed, washing the household utensils with ash, and frequent dipping of hands in soap water; all of which enhance the chances of fungal infection.
Laboratory Diagnosis of Epidermophyton infections
Specimen: Skin scrapings, nail scrapings
Microscopic Examination
10–20% potassium hydroxide, with or without calcofluor white, and the specimens skin or nails.
Cultural Examination
Using inhibitory mold agar or SDA medium containing cycloheximide and chloramphenicol which suppresses mold and bacterial growth. Colonies are usually slow-growing, greenish-brown or khaki-colored with a suede-like surface, raised and folded in the center, with a flat periphery and submerged fringe of growth.
LPCB Tease mount for Microscopic appearance
The colony is teased and an LPCB mount is made to demonstrate the hyphae and spore ( conidia). Conidia is of two types i.e. Microconidia:- small unicellular
Macroconidia:- Multicellular, septate
Special hyphae:- hyphae such as racquet hyphae as shown above picture.
Biochemical test
Urease test:- Epidermophyton species are urease negative
Other methods of diagnosis
Hair perforation test:- fungi pierce hair-producing wedge-shaped perforations.
Positive:- Trichophyton mentagrophytes and Microsporum canis.
Negative: Epidermophyton species
Molecular methods
PCR assay: A variety of PCR assays are applicable, among them some are-
- PCR-Restriction Fragment Length Plormophism (PCR-RFLP) is used to distinguish the 12 species that cause dermatophytosis infections.
- Real-time PCR is also used to identify E. floccosum after fungal lysis
Treatment of Epidermophyton infections
Tinea pedis, tinea cruris, and tinea corporis are treated tropically using naftifine, terbinafine, butenafine, clotrimazole, ketoconazole, econazole miconazole, sulconazole, oxiconazole, cyclopyrox, and tolnaftate while
tinea unguium or onychomycosis(nail infections), topical therapy is normally unsuccessful, and thus, the use of systemic oral therapy for a prolonged period is advised.
Prevention and control of Epidermophyton infections
- Avoiding touching lesions on the skin and nails of infected people.
- Wearing loose-fitting clothing on the affected areas.
- Practicing good hygiene when engaging in activities that involve close human contact with skin
Key Notes
- Trichophyton rubrum is the commonest causative agent of dermatophytoses worldwide.
- Trichophyton species may cause invasive infections in immunocompromised hosts.
- Antropophilic species are highly contagious between humans
- Males more commonly infected as progesterone is inhibitory to dermatophyte growth.
Sporothrix: Introduction, Morphology, Pathogenesis, Complications, Laboratory Diagnosis, Treatment, Prevention, and Control
Introduction of Sporothrix schenckii
Sporothrix schenckii is a thermodimorphic fungus widely is widely distributed in soil and in living as well as dead decaying environments. It is a causative agent of sporotrichosis, in both animals and humans. Sporotrichosis is a subacute or chronic infection and a cosmopolitan disease, occurring preferably in tropical and subtropical regions. Infection may also be associated with ulcerative, and suppurative lesions with skin and lymphatic system forming nodular lesions. Sporotrichosis was described for the first time by Benjamin Schenck, who was a medical student at Johns Hopkins Hospital in Baltimore in 1898. After the etiological isolation, Schenck sent the sample to the mycologist Erwin Smith who concluded that the agent was a microorganism of the genus Sporotrichum. The disease was reported for the second time in 1900 by Hektoen and Perkins, who classified the etiological agent as S. schenckii, with the pathogen isolated from the specimen suctioned from the skin lesions of the patient (Hektoen and Perkins 1900). The disease is also known as Rose Gardener’s disease.
Taxonomic Classification of Sporothrix schenckii
(described by Hektoen and Perkins in 1900)
Kingdom: Fungi
Phylum: Ascomycota
Class: Euascomycetes
Order: Ophiostomatales
Family: Ophiostomataceae
Genus: Sporothrix
Species: S. schenckii
Note: New clinically significant species are Sporothrix brasillensis, Sporothrix globosa and Sporothrix mexicana . A study has proposed Sporothrix schenckii var. luriei to species level (Sporothrix luriei).
Habitat of Sporothrix schenckii
It is commonly found in soil and on living plants such as barberry shrubs and roses or in plant debris such as pine bark mulch and sphagnum moss.
People having professions like florists, gardeners, and forestry workers are prone to infection and infection is common in tropical and subtropical regions.
Morphology of Sporothrix schenckii
S. schenckii is a dimorphous fungus. It has a filamentous form in the basic mycological culture at 25°C, consisting of hyaline, septate hyphae 1 to 2 μm in width. Fungal growth in the colonies is characterized by branched septate hyphae, which produce small distinct asexual spores known as 3-5 μm conidia, which are brown in color. Conidia are produced by conidiophores that arise from the septate hyphae at right angles. The conidiophore, at the ends, is tapered. The conidia formed are lustered on tiny denticles at the apex of the conidiophore forming a flower-like appearance. Conidia are ovoid or elongated, 3-6 x 2-3 μm, hyalin, single-celled and smooth-walled. Large single occurring conidia may also be formed as fungal culture ages. The large conidia are dark, thick, obovate to angular. Budding yeast cells are fusiform on enriched medium approximately 1–3 × 3–10 μm, spherical or oval-shaped, are formed.
Cultural Characteristics of Sporothrix schenckii
Routine mycological agar media, such as malt extract agar and potato dextrose agar, grow slowly at room temperature of 25°C, forming blackish/greyish and shiny colonies, which mature into moist, glabrous, wrinkled, and fuzzy colonies, colored in black. In a rich medium such as the brain heart infusion media (BHI) at 35-37°C, S. schenckii is thermally dimorphic, the growth of which is characterized by multiple budding yeast cells. Colonies are produced in the BHI medium that is glabrous, white-greyish to yellowish in color, yeast cells.
Transmission of Sporothrix schenckii
The inoculation of the fungus into the host is caused by the traumatic implantation of the fungus from contaminated soil, plants, and organic matter with fungus. It’s common in florists, gardeners, miners, and forest workers.
Zoonotic transmission from cats has also been reported in Brazil in a few cases. It has an incubation period of 1-12 weeks.
Pathogenesis of Sporothrix schenckii
Virulence Factors of Sporothrix schenckii are proteinases, melanin, and adherence molecules.
Proteinases: It Produces two extracellular proteinases, proteinase I and II.
Thermotolerance S. schenckii has the ability to grow at temperatures between 35-37°C. At 35°C, it can cause lymphatic sporotrichosis and at 37°C, it can cause disseminated and extracutaneous lesions of sporotrichosis.
Melanin synthesis: Melanin, an insoluble compound that is synthesized by Sporothrix schenckii has been associated with virulence in many fungal groups. Melanin is found on the dematiac conidia of the fungus
Conidial melanization increases the resistance of Sporotrix schenckii to macrophageal phagocytosis, which initiates the first stage of conidia spores infection, the infective fungal particle. Melanin pigmentation plays a key role in causing skin sporotrichosis by increasing the invasiveness of the fungus to the host.
Adherence: Sporotrix schenckii contains adhesives such as integrins and adhesin lectin-like molecules that are capable of detecting glycoproteins on the extracellular matrix of the skin tissues, i.e. fibronectin, laminin, and type II collagen. Fibronectin adhesives are located on the surface of the yeast cells and are attributed to the host by fungal adhesion factors.
Laminin receptors are located on the hyphae fungus and the yeasts that have the ability to bind to the extracellular matrix.
The presence of these adhesives promotes the adherence of the fungus to the host tissues and enhances the spread of the disease.
Clinical Manifestations of Sporotrichosis
Infection with lymphoma
This is the most common manifestation of S. schenckii. Lesions occur in the hands and arms, lower extremities, trunk, and face. Primary lesions occur within the first few weeks of inoculation. Lesions are small nodules that progress towards ulceration and are a bit painful and unprejudiced.
New nodules spread to the lymphatics and become ulcerated. Lymphangitis can develop, making the lymph nodes swollen and painful.
Cutaneous infections
S. schenckii is a common causative agent for suppurative and granulomatous inflammatory reactions in the dermis and subcutaneous tissues. It is characterized by microabscess and fibrosis, hyperkeratosis, parakeratosis, and pseudoepithelioma hyperplasia.
Fixed Cutaneous Infection
Normally, this is the development of a single lesion on the face. The lesion can
be ulcerated or verrucous and it only disappears with antifungal therapy.
Osteoarticular sporotrichosis
It is a rare condition of sporotrichosis, which commonly affects alcoholics.
It is characterized by septic arthritis caused by traumatic inoculation that spreads to the joints. Bursitis and tenosynovitis may occur with carpal tunnel syndrome.
Pulmonary sporotrichosis
It is a subacute to chronic infection and is common in people with chronic obstructive pulmonary disease (COPD). It occurs after conidia are inhaled. It is characterized by fever, fatigue, weight loss, coughing, sputum production, and hemoptysis. Infected individuals often develop chronic cavitary pulmonary histoplasmosis, tuberculosis, or atypical mycobacterial infection.
Clinical classification of sporotrichosis
Skin
- Lymphocutaneous
- Fixed cutaneous
- Multiple inoculations
Mucous membrane
- Ocular
- Nasal
- Others
Systemic
- Osteoarticular
- Cutaneous disseminated
- Pulmonary
- Neurological
- Other locations/sepsis
Immunoreactive
- Erythema nodosum
- Erythema multiforme
- Sweet’s syndrome
- Reactive arthritis
Spontaneous regression
Laboratory Diagnosis of Sporotrichosis
Specimen
It depends on the site of infections and may be tissue biopsy, pus from lesions, sputum, urine, blood, CSF, synovial fluids, etc.
Direct Examination
KOH mount: Presence of yeast cells and an elongated cigar-shaped morphology
Gram stain: Gram-Positive yeast cells
Histological Examination: For this purpose following stains may be used-
Hematoxylin and eosin stain, Gomori methenamine silver (GMS), and Periodic acid-Schiff (PAS) stain.
Cultural Characteristics
SDA: Sabouraud dextrose agar (SDA) with chloramphenicol and on media with cycloheximide ( actidione), growth occurs in 5-7 days at 25°C, forming filamentous hyaline colonies which become dark at the center as the culture ages. Dematiaceous conidia are also observed as shown above image ( few at center)
PDA and CMA: Potato dextrose agar (PDA) and cornmeal agar (CMA)are used to show conidiogenesis.
Dimorphic media: Brain heart infusion agar, chocolate agar, and blood agar are used to show dimorphism at 35 to 37°C. Growth of colonies develops within 5-7 days to form yeast cell colonies which are creamy and yellow, to tan color.
LPCB Preparation
There is the presence of a filamentous state with thin hyphae and denticle microconidia resembling daisy flowers from the culture obtained at 25°C.
Molecular Detection
Detection of PCR amplicons: Oligonucleotide primers for differentiation of S. schenckii from other fungal species.
Serological Test
Antibody detection: Tube agglutination test to detect agglutinin antibodies (high titers)
Antigen detection: Latex Agglutination in which antigen-coated latex particle is used to detect for sporotrichin antigen in the sera of infected patients.
Sporotrichin Skin Test
This skin test is used to detect cell-mediated immune response also called delayed hypersensitivity and it is useful for confirming the present and previous infection by S. schenckii.
Treatment of Sporotrichosis
A saturated solution of potassium iodide is given as oral administration whereas itraconazole is currently the first-choice treatment. For the treatment of systemic infection amphotericin B can be used.
Prevention and Control of Sporotrichosis
Installation of protective clothing such as gloves and long sleeves during high-risk activities, e.g. handling sphagnum moss, wires, rose bushes, hay bales, conifer (pine) seedlings, or other materials that may facilitate exposure to the fungus. Cats with sporotrichosis should be treated correctly and kept isolated in a proper place to prevent zoonotic transmission.
Key Notes on Sporothrix schenckii
- S. schenckii var. luriei differs from S. schenckii by producing large, often septate, budding cells and not assimilating creatine and creatinine.
- The sporotrichin skin test is used to confirm the diagnosis of bulbar conjunctival sporotrichosis.
- The most commonly used dimorphic medium is brain heart infusion (BHI) agar with blood.
Histoplasma: Introduction, Morphology, Life Cycle, Pathogenesis, Laboratory Diagnosis, Treatment and Prevention
Introduction of Histoplasma capsulatum
Histoplasma capsulatum was discovered by Pathologist, Samuel Taylor Darling in 1906. It is a causative agent of histoplasmosis ( systemic disease) sometimes called Darling’s disease, in honor of the discoverer. It is a dimorphic fungus and thus it grows as filamentous molds as saprobes and in the culture at 25°C; while in humans or culture at 37 °C, it transforms to a unicellular morphology ( yeast cells).
Taxonomic classification
(Darling, 1906)
Kingdom: Fungi
Phylum: Ascomycota
Subphylum: Ascomycotina
Class: Ascomycetes
Order: Onygenales
Family: Onygenaceae
Genus: Histoplasma
Species: Histoplasma capsulatum
The genus, Histoplasma contains one species, H. capsulatum, H. capsulatum has two varieties: Histoplasma capsulatum var. capsulatum and H. capsulatum var. duboisii ( causative agent of African histoplasmosis)
Habitat:
Soil contaminated with bird droppings or excrement of bats is the common natural habitat for Histoplasma. Although it is claimed to exist worldwide, tropical areas are where this fungus is more frequently encountered. It is endemic in Midwestern and Central USA, along the Mississippi and Ohio river valley.
Morphology of Histoplasma
It completes in two phases i.e. macroscopic and microscopic features.
Macroscopic Features
Being a thermally dimorphic fungus, Histoplasma capsulatum grows in mold form at 25°C, and in yeast form at 37°C. Below are the macroscopic characteristics at varying temperatures and for both varieties.
At 25°C
Colonies are slow-growing and granular to cottony in appearance. The color is white initially and usually becomes buff-brown with age. The colonies are not sensitive to cycloheximide ( actidione) in the culture media. From the reverse, a yellow or yellowish-orange color may be observed. While these features are best observed on SDA, brain heart infusion agar (BHIA) enhances growth more efficiently.
At 37°C
Creamy, slowly growing, moist, and yeast-like colonies are formed. This phase is observed in infected tissues and in vitro on enriched media, such as BHIA containing 5-10% blood.
For definitive identification of the fungus, yeast-to-mold conversion should be demonstrated.
Microscopic Features
At 25°C
Hyphae are septate and hyaline. H. capsulatum produces hyphae-like conidiophores which arise at right angles to the parent hyphae. It has both macro-and microconidia. Macroconidia are tuberculate, thick-walled, round, unicellular, hyaline, large, and often have fingerlike projections on the surface. These macroconidia are also referred to as tuberculochlamydospores or macroaleurioconidia. Microconidia (microaleurioconidia) are unicellular, hyaline, and round, with a smooth or rough wall.
At 37°C
Narrow-based, ovoid, budding yeast cells are formed. Yeasts of H. capsulatum var. capsulatum are smaller than (2-4 µm) those
Life cycle of Histoplasma
Histoplasma spores circulate in the air after contaminated soil is disturbed. The spores are too small to see without a microscope. When people breathe in the spores, they are at risk of developing histoplasmosis. After the spores enter the lungs, the person’s body temperature allows the spores to transform into yeast. The yeast can then travel to lymph nodes and can spread to other parts of the body through the bloodstream. Histoplasmosis is not contagious but is contracted by inhalation of the spores from disturbed soil or guano. The inoculum is represented principally by microconidia. These are inhaled and reach the alveoli. In the alveoli, macrophages ingest these microconidia. They survive inside the phagosome. As the fungus is thermally dimorphic, these microconidia are transformed into yeast. They grow and multiply inside the phagosome. The macrophages travel in lymphatic circulation and spread the disease to different organs. Within the phagosome, the fungus has an absolute requirement for thiamine. Cell-mediated immunity for histoplasmosis develops within 2 weeks. If the patient has strong cellular immunity, macrophages, epithelial cells, and lymphocytes surround the organisms and contain them, and eventually calcify. Fibrosis around the cavities in the lung creates dense, round, flattened areas that are described as “coin lesions”. In immunocompromised individuals, the organisms disseminate to different organs such as bone, spleen, liver, adrenal glands, and mucocutaneous membranes, resulting in progressive disseminated histoplasmosis. Chronic lung disease can manifest.
Pathogenesis of Histoplasma
Histoplasmosis is a systemic intracellular mycotic disease caused by dimorphic fungus, H. capsulatum. It usually occurs by the inhalation of microconidia by the host, deposit in alveoli, and rapidly convert to a parasitic yeast form in tissues. This germination and conversion can occur prior to or after ingestion by pulmonary macrophages. Conidia and yeasts are ingested by macrophages and reticuloendothelial cells where the organism can survive within phagolysosomes. Once within the macrophage, the yeast multiply and travel to hilar and mediastinal lymph nodes where they gain access to the blood circulation for dissemination to various organs. It is also known as Cave disease or Darling’s disease or Ohio valley disease or reticuloendotheliosis or spelunker’s lung and caver’s disease. It is common among AIDS patients because of their suppressed immunity and approximately 30% of HIV/AIDS patients diagnosed with histoplasmosis die from it. In immunocompetent individuals, past infection results in partial protection against ill effects if reinfected.
Types of Histoplasmosis
It may be divided into the following types:
- Primary pulmonary histoplasmosis
- Progressive disseminated histoplasmosis
- Primary cutaneous histoplasmosis
- African histoplasmosis
Clinical Findings
The clinical findings of histoplasmosis range from asymptomatic infection to disseminated sepsis. These manifestations depend mainly on the magnitude of exposure (i.e. the number of fungal particles inhaled), the immunological status of the host, and the virulence of the infective strain, indicating that environmental and genetic factors control the manifestation of the disease. Additionally, in the setting of severely immunocompromised patients, such as individuals with AIDS, H. capsulatum strains previously not considered virulent have been able to cause fatal disease. In patients who are clinically ill, histoplasmosis generally occurs in one of three forms-acute pulmonary, chronic pulmonary, and disseminated. There is generally a complete recovery from the acute pulmonary form (another “flu-like” illness). However, if untreated, the disseminated form of the disease is usually fatal. Patients will first notice shortness of breath and a cough which becomes productive. If symptoms of histoplasmosis infection occur, they will start within 3 to 17 days after exposure; the average is 12–14 days. Most affected individuals have clinically silent manifestations and show no apparent ill effects. The acute phase of histoplasmosis is characterized by non-specific respiratory symptoms, often cough or flu-like. Chest X-ray findings are normal in 40–70% of cases.
Chronic histoplasmosis cases can resemble tuberculosis.
- The sputum may be purulent or bloody.
- Patients will become anorexic and lose weight.
- Complain of fever and chills
- Night sweats
- Fatigue (extreme tiredness)
- Headache and body aches
- Chest pain
This again sounds like tuberculosis, and the lung X-ray also looks like tuberculosis, but today radiologists can distinguish between these diseases on the chest film (histoplasmosis usually appears as bilateral interstitial infiltrates.)The skin test is NOT used for diagnostic purposes, because it interferes with serological tests.
While histoplasmosis is the most common cause of mediastinitis, this remains a relatively rare disease. Severe infections can cause hepatosplenomegaly, lymphadenopathy, and adrenal enlargement. Lesions have a tendency to calcify as they heal.
Laboratory Diagnosis of Histoplasma
To diagnose histoplasmosis medical and travel history, Signs and symptoms, physical examinations, and laboratory tests are useful.
Specimen
Clinical specimens depend on the nature of the infection. e.g. Sputum or Bronchial alveolar lavage: if it is a pulmonary disease, or Skin scrapping from the edges of ulcer. Biopsy material from the diseased organ. Aspirated Pus from lymph nodes and subcutaneous abscess. Bone marrow is an excellent source of fungus, which tends to grow. Peripheral blood is also a source of visualizing the organism histologically. The yeast is usually found in monocytes or in PMN’s. In peripheral blood, H. capsulatum appears as a small yeast about 5-6 µ in diameter. (Blastomyces is 12 to 15 µ). Gastric washings are also a source of H. capsulatum as people with pulmonary disease produce sputum and frequently swallow their sputum.
CSF and Urine for culture
Microscopy
H. capsulatum is most likely to be found within cells instead of free in the fluid. Staining – Wright’s method, Giemsa stain, or Calcofluor white and special stains: Papanicolaou stain or H & E stain for tissue specimen. Mucus and other thick substances should be treated with N-acetyl-L-cysteine before the wet mount is prepared. KOH wet mount prepared from clinical samples shows tiny yeast cells.
Culture
Blood agar or Modified SDA with antimicrobials and actidione ( cycloheximide)
BHIA containing 5-10 % blood is strongly recommended for specimens from sites that are sterile.
Yeast extract phosphate agar with ammonium hydroxide: It encourages conidiation and inhibits Candida species. Potato dextrose agar (PDA): to encourage conidia production.
All cultures should be examined daily for 1 week. When it is grown on modified Sabouraud dextrose agar (SDA) with antimicrobial at 25ºC, it appears as a white, cottony mycelium after 2 to 3 weeks. Later they become woolly, as aerial hyphae develop. The mold phase of H. capsulatum is characterized by thin, branching, septate hyphae, 1-2 µm, that produce microconidia (2-5µm). Macroconidia develop directly on the hyphae or on short, slender conidiophores that develop at right angles to vegetative hyphae. The macroconidia are hyaline, unicellular, and relatively large 8-14 µm, spherical to pyriform shape. As the macroconidia age, they become tuberculate macroconidium, they form finger-like extensions of the thick wall of conidium.
Mature macroconidia sometimes described
Grown at 37ºC the budding yeast form appears after 10-15 days. It is a white to the tan and mucoid colony, with a rough membranous texture. The yeast cell is 5-6 µ in diameter and slightly oval in shape and found exclusively within macrophages. To confirm the diagnosis, one must convert the organism from mycelium to yeast or use the DNA probe. Primary cultures for H. capsulatum should be held for 10-12 weeks before being discarded as no Growth. Culture of H. capsulatum on SDA showing a white suede-like colony with a pale yellow-brown. Microscopic morphology of the saprophytic or mycelial form of H. capsulatum showing characteristic large, rounded, single-celled, tuberculate macroconidia formed on short, hyaline, undifferentiated conidiophores. Yeast-like culture of H. capsulatum on blood agar incubated at 37°C.
Non-culture based diagnosis of histoplasmosis
These tests include antibody and antigen detection as well as newer
techniques that have been developed in molecular diagnosis for the improved identification of H. capsulatum in clinical specimens.
Antibody detection
The two routine methodologies are complement fixation and immunodiffusion. Latex agglutination can also be used.
Immunodiffusion Assay: The Histoplasma immunodiffusion assay utilizes 2 different antigens, the H and the M proteins, and we can therefore detect up to 2 different precipitating bands in the agar plate. Antibodies to the M antigen, appear shortly the following exposure and can remain detectable by immunodiffusion for up to 3 years following disease resolution. Therefore, the presence of the M band alone cannot be used to discriminate between acute or remote infection. On the other hand, the presence of an H band either alone or in combination with the M band is indicative of active or recent histoplasmosis because the H band of the immunodiffusion test is usually present for only 4 to 6 weeks after exposure. Importantly, a negative immunodiffusion result should not be used to exclude histoplasmosis as the specimen may have been collected early following exposure and prior to the development of detectable precipitating antibodies. Specificity >95% and variable sensitivity 80- 100 %.
Complement Fixation Test: Alongside immunodiffusion, there is also Histoplasma complement fixation testing. Briefly, complement fixation assays are based on the ability of antibody-antigen complexes, formed between antibodies present in the patient sample and added fungal antigens, to fix and inactivate exogenously added complement. Sensitized RBCs are added to this mix and since the complement pathway is inactivated, the red blood cells will remain intact and settle to the bottom of the well as a compact pellet, indicative of a positive reaction and the presence of specific antibodies. In the absence of patient antibodies, however, the added complement will remain active and lead to lysis of the sensitized red blood cells. Therefore, the presence of lysis is indicative of a negative reaction and the absence of specific antibodies. The Histoplasma complement fixation assay is performed using 2 different Histoplasma antigens, one purified from the yeast phase and the second purified from the mycelial phase of growth. Serial dilution of patient samples, allows us to determine an endpoint titer that can be used to guide diagnosis. For either antigen, serially increasing titers or an endpoint titer greater than or equal to 1:32 is associated with the presence of active histoplasmosis. A titer of 1:8 or 1:16 is considered positive, but only presumptive evidence of infection and additional clinical correlation is necessary. Titers less than 1:8 are not considered to be significant and therefore are not reported. Importantly, low-level titers may be detected in individuals who reside in endemic areas and are otherwise healthy. Finally, declining complement fixation titers over months to years have been associated with disease resolution, but should not be used to monitor response to therapy.
The complement fixation assay has a higher sensitivity for histoplasmosis as compared to immunodiffusion assays, particularly during early disease, however, their specificity is lower and for this reason, complement fixation and immunodiffusion are performed concurrently. Also, these assays are fairly labor-intensive with long incubation times which leads to an average turnaround. One disadvantage is that complement-fixing antibody develops late in the disease, about 2 to 3 weeks after onset. A second disadvantage is that it cross-reacts with other mycotic infections like blastomycosis, paracoccidioidomycosis . An advantage of the C-F test is that it is quantitative, so the physician can follow the course of the disease by observing the titer of several samples.
Antigen detection
Antigen detection tests may be more effective than antibody testing for diagnosing histoplasmosis.
- Radioimmunoassay (RIA): Recently, a radioimmunoassay can be used to measure H. capsulatum polysaccharide antigen (HPA) levels in samples of a patient’s urine, serum, and other body fluids. The test appears to meet the important need for a rapid and accurate method for early diagnosis of disseminated histoplasmosis, especially in patients with AIDS. HPA is detected in body fluid samples of most patients with disseminated infection. The antigen cross reacts with Coccidioides immitis and Blastomyces dermatidis. High Sensitivity and low specificity, so should be confirmed by another method. Immy exoantigen immunodiffusion test kit for the identification of H. capsulatum, C. immitis, and B. dermatitidis. Exoantigen immunodiffusion plate showing positive identification of H. capsulatum.
- ELISA
Exoantigens
Exoantigens are valuable for the immunoidentification of fungal pathogens and for resolving taxonomic problems. Most fungi produce unique antigens that allow specific identification. Exoantigens are soluble immunogenic macromolecules produced by fungi early in their development.
Molecular based methods
As described, H. capsulatum colonies grow slowly and the differentiation from other dimorphic or saprophytic fungi having similar colony or microscopic morphology is challenging. In order to confirm the diagnosis, tests for the production of exoantigens and the conversion to the yeast phase should be done, which takes at least 3 to 4 weeks. Methods for the identification of fungal isolates such as species-specifics DNA can decrease this time-consuming step while maintaining or improving the specificity, accuracy, and sensitivity. The use of chemiluminescence-labeled DNA probes for the detection of specific sequences of rRNA has been able to detect and confirm H. capsulatum clinical isolates tested. PCR Assays: A real-time PCR assay that correctly identified H. capsulatum from among a variety of fungi growing in the laboratory appears promising. Using this assay, positive identification of H. capsulatum was shown in tissue biopsies and bronchoalveolar lavage fluid from patients who had documented histoplasmosis. Two different semi-nested PCR assays have also shown promise when applied to blood and tissue scrapings obtained from patients with histoplasmosis and mice experimentally infected with H. capsulatum
Histoplasmin skin test: A person can learn from a histoplasmin skin test whether he or she has been previously infected by H. capsulatum. This test, similar to a tuberculin skin test like delayed-type hypersensitivity. Skin test with intradermal injection of 0.1ml of Histoplasmin antigen, a culture filtrate of the mycelial phase of growth, in the forearm. It is useful as an epidemiological tool. A histoplasmin skin test becomes positive 2 to 4 weeks after a person is infected by H. capsulatum. The test is positive after 48 hours with induration of 5 mm. A positive skin test does not mean that a person is completely protected against ill effects, it indicates either present or past exposure to H.capsulatum. A previous infection by H. capsulatum can provide partial protection against ill effects if a person is reinfected.
Animal Pathogenicity: Dogs, guinea pigs, hamsters, rabbits, mice. The mouse is an ideal laboratory animal for the isolation of H. capsulatum. Mycelial and yeast forms can be inoculated to establish systemic infection. Due to the discovery of newer methods, its frequency of usage has decreased.
Treatment
For some people, the symptoms of histoplasmosis will go away without treatment. However, prescription antifungal medication is needed to treat severe histoplasmosis in the lungs, chronic histoplasmosis, and infections that have spread from the lungs to other parts of the body (disseminated histoplasmosis). The drug of choice is amphotericin (avoided in renal patients due to the risk of nephrotoxicity. Itraconazole and Voriconazole are now also being used. Liposomal preparations of amphotericin B are more effective than deoxycholate preparations. Alternatives to itraconazole are posaconazole, voriconazole, and fluconazole. Individuals taking itraconazole are monitored for hepatic function.
Epidemiology of Histoplasma
H. capsulatum is found throughout the world. It is endemic in certain areas of the United States, particularly in states bordering the Ohio River valley, the lower Mississippi River, and the Missouri River. The humidity and acidity patterns of soil are associated with endemicity. Incidence of histoplasmosis in adults aged 65 years and older in the U.S. to be 3.4 cases per 100,000 population. Rates were highest in the Midwest, with an estimated 6.1 cases per 100,000 population. Bird and bat droppings in soil promote the growth of H.capsulatum. Contact with such soil aerosolizes the microconidia, which can infect humans. It is also common in caves in southern and East Africa. Positive histoplasmin skin tests occur in as many as 90% of the people living in areas where H. capsulatum is common, such as the eastern and central United States. In Canada, the St. Lawrence River Valley is the site of the most frequent infections, with 20-30 percent of the population testing positive. In the United States, an estimated 60% to 90% of people who live in areas surrounding the Ohio and Mississippi River valleys (where H. capsulatum is common in the environment) have been exposed to the fungus at some point during their lifetime India is another Asian country where H. capsulatum is known to be endemic, although the true prevalence of this mycosis is still underappreciated. The first case was reported as early as 1954, and since then several cases have been published. In India, the majority of histoplasmosis cases were reported from the eastern and north-eastern parts of the country, especially from Calcutta (West Bengal) and Assam. The Gangetic West Bengal is the site of most frequent infections, with 9.4 percent of the population testing positive. H. capsulatum was isolated from the local soil proving the endemicity of histoplasmosis in West Bengal. The prevalence of histoplasmosis has not been well studied in Nepal.
Prevention
It can be difficult to avoid breathing in H. capsulatum in areas where it’s common in the environment. In areas where Histoplasma is known to live, people who have weakened immune systems (for example, by HIV/AIDS, an organ transplant, or medications such as corticosteroids or TNF- inhibitors) should avoid doing activities that are known to be associated with getting histoplasmosis, including Disturbing material (for example, digging in the soil or chopping wood) where there are bird or bat droppings, cleaning chicken coops, exploring caves.
Key Notes on Histoplasma
- Bone marrow biopsy for histopathology may be the most rapid method of establishing a definitive diagnosis of invasive infection.
- The structure of the H. capsulatum yeasts is very similar to other pathogens(Candida glabrata, Penicillium marneffei, Pneumocystis jirovecii, Toxoplasma gondii, Leishmania donovani and Cryptococcus neoformans) and thus it is extremely important to be familiar with the morphology of these pathogens and the staining characteristics of the yeast by different methods.
- This African variant differs by having larger (7-15 µm) budding yeast cells in-vivo.
- In people who have weakened immune systems, histoplasmosis can remain hidden in the body for months or years and then cause symptoms later (also called a relapse of infection).
- Histoplasmosis can’t spread from the lungs between people or between people and animals. However, in extremely rare cases, the infection can be passed through an organ transplant with an infected organ.
- Systemic Mycoses : H. capsulatum is also a fungus which is responsible for system mycoses other than B. dermatitidis, P. barasiliensis, C.immitis, C. neoformans. Four of these pathogens (H. capsulatum, B dermatitidis, P. barasiliensis and C. immitis) are dimorphic. They grow as filamentous molds in the culture at 25°C and yeast forms in humans or culture at 37°C.
- Differential Diagnosis: Tuberculosis, localized pneumonitis is mistaken for Mycoplasma pneumoniae, Legionella, Coxiella burnetii, and Chlamydia pneumoniae. The uninucleate yeasts should be distinguished from other intracellular organisms like Leishmania donovani ( contain kinetoplast), Toxoplasma gondii (tachyzoites being protozoa are not stained with GMS stain). P. marneffei (dividing transverse fission cells), C. neoformans (capsulated), Candida glabrata (never found intracellularly).
- Sources of Histoplasmosis: H. capsulatum is found in blackbird roosts, chicken houses, and bat guano and especially from the soil with a high nitrogen content resulting from deposits of excreta from chicken, starlings, and bats. It is a significant occupational disease in bat caves in Mexico when workers harvest the guano for fertilizer. Disruption of soil from excavation or construction can release infectious elements that are inhaled and settle into the lung. In the endemic area, the majority of patients who develop histoplasmosis (95%) are asymptomatic. 5 % of the cases have chronic progressive lung disease, chronic cutaneous or systemic disease, or an acute fulminating fatal systemic disease. All stages of this disease may mimic tuberculosis and thus diagnosis is made from their history, serologic testing, or skin test.
- Most of the infections associated with histoplasmosis are asymptomatic. even though the common symptoms of acute and epidemic histoplasmosis include high fever, cough, and asthenia, and weight loss.
- People with compromised immune systems such as HIV/AIDS, cancer, and organ transplant recipients are at risk of developing this disease.
- Unlike its name; H. capsulatum is not encapsulated. The designation H. capsulatum is actually a misnomer (inaccurate name).
- Tuberculate macroconidium (with typical thick walls and radial, finger-like projections) is a diagnostic structure of H. capsulatum.
Blastomyces dermatitidis: General Characteristics, Pathogenesis, Clinical Findings, Laboratory Diagnosis, Epidemiology, Prevention, and Control
Introduction of Blastomyces dermatitidis
Blastomyces dermatitidis is a thermally dimorphic fungus that causes blastomycosis, a lung infection that is chronically invasive and spreads into the skin and bones, affecting both humans as well as animals.
Taxonomic Classification
(Described by van Tieghem in 1876)
Kingdom: Fungi
Phylum: Ascomycota
Class: Euascomycetes
Order: Onygenales
Family: Onygenaceae
Genus: Blastomyces
Species: Blastomyces dermatitidis
Habitat of Blastomyces dermatitidis
Naturally, B. dermatitidis are present in soil and organic matter such as animal waste, fragments of plants, leftover insects, and dust. It thrives in cold, dark areas with organic debris and a pH value of 6.0. In North America, including the USA and Canada, they are prevalent, especially in the Mississippi, Ohio, and Missouri valleys that have the highest occurrences of blastomycosis infections.
Morphology and Cultural characteristics of Blastomyces dermatitidis
Blastomyces dermatitidis are dimorphic fungi that grow both as molds and as yeast at 37 ° C, forming asexual spores known as conidia or large chlamydospores. When the fungi are growing in mycological cultures, mold forms are produced, while the yeast forms are produced when the fungi grow in host tissues and some specialized culture media. At room temperature, Blastomyces dermatitidis grows on the Sabouraud agar, developing white or brownish colonies. The branched hyphae that hold spherical, ovoid, or piriform conidia (3-5 μm in diameter) are composed of these colonies. The slender terminal or lateral conidiophores are retained by the conidia. Larger chlamydospores (7–18 μm) may also be formed in SDA media. Blastomyces dermatitidis develops as a thick-walled, multinucleated, spherical yeast (8–15 μm) forming single buds in host tissues or culture at 37 °C. With a large foundation, the bud and the parent yeast are attached, and the bud sometimes grows to the same size as the parent yeast until they become separated. The colonies of yeast are wrinkled, waxy, and soft.
Pathogenesis of Blastomyces dermatitidis
Virulence Factors of Blastomyces dermatitidis
The fungi are known to generate a known weak Blastomyces dermatitidis antigen. As blastomycin, with signs that can be identified with complement fixation. Large titers of complement fixation in patients with infections with blastomycosis are seen. The fungal infection also causes antibodies to be developed in antisera. The antigen of B. dermatitidis is known as antigen A. In causing the fungal infection, these antigens were related to the virulence of the fungi, although there is no evidence to support the levels of virulence induced by these antigens. The fungi generate small blastospores that are light and thick-walled, allowing them to be easily transported by air and thus easily inhaled. The thick-walled fungal spores cause the host tissues to be easily adhered to and colonized.
Transmission of Blastomyces dermatitidis
Transmission of B. dermatitidis spores by inhalation through the lungs, from dirty soil and debris. Spore exposure is common from excavation, building, digging, or airborne exposure is due to clearing wood. Fungal spores seldom invade wounds that are open. There is no evidence of human-to-human or animal-to-human transmission.
Clinical Features of Blastomycosis
When blastospores are inhaled into the lungs, the initial infection begins, spreading quickly to the skin and other areas of the body. Pulmonary infiltration, associated with acute lower respiratory infections, including fever, malaise, night sweats, cough, and myalgia, is the most common clinical manifestation. Chronic pneumonia may also be present in patients. Different pyogranulomatous reactions to neutrophils and non-caseating granulomas are shown by histological proof. The development of skin lesions that can develop to ulcerated verrucous granulomas with bordering and centralized scarring shows dissemination on the skin. Microabscesses with rough sloping edges fill the boundaries of the skin lesions. Diseases can also spread to the bones and genitals (prostate, epididymis, and testis) and to the central nervous system. In three clinical types, the disorder blastomycosis occurs:
- cutaneous disease
- pulmonary disease
- disseminated disease
Laboratory Diagnosis of Blastomycosis
Specimen: It depends on the site infection and the most common specimens are sputum, pus, and tissue biopsies.
Direct Examination
Potassium hydroxide (KOH) mount: It shows the presence of yeast cells, which are 3-5 µm in diameter.
Culture Characteristics
Blastomyces dermatitidis are dimorphic fungi that grow both as molds ( 25°C) and as yeast at 37 ° C. Sabouraud Dextrose Agar (SDA) forms white or brownish colonies at room temperature ( ( 25°C) as shown above image.
LPCB preparation
LPCB preparation from the plate incubated at 37°C shows yeast-like cells which are thick-walled, multinucleated, and spherical shaped while from the plate incubated at 25°C shows one-celled, smooth-walled conidia borne on short lateral to terminal hyphal branches as shown above images.
Histological Examination
Following stains are useful for identifications of this fungus and they are-
Hematoxylin and Eosin(H &E) stain: It uses to observe neutrophils and pyogranulomatous reactions due to neutrophilic interactions of the granuloma from the host tissues. Hematoxylin stains the nuclei of cells blue to bluish-purple, and eosin stains the cellular elements in the tissues from pink to red as shown above picture.
Gomori’s methenamine silver stain (GMS): It stains the yeast cell wall deep black and the interior of the yeast cells are rose-colored, while the background is green.
Periodic acid-Schiff (PAS) Stain: It stains the yeast cells red with a pink background or light green, identified by the type of counterstain that is used. The histological stains may show small yeast cells (2-10 µm in diameter) to large yeast cells (25-40 µm in diameter) with hyaline short septate hyphae.
Immunological Diagnosis
Complement fixation test (CFT) for detecting the blastomycin antigen; high CF titers indicate the presence of Blastomyces dermatitidis antigen. Immunodiffusion is used for the detection of B. dermatitidis antigen A. Skin test for detection of blastomycin. ELISA for detection of antibodies against B. dermatitidis antigen A.
Treatment of Blastomycosis
Amphotericin B, itraconazole, or ketoconazole are the drugs of choice for treatment. Amphotericin B should be preferred particularly in immunocompromised patients while mild pulmonary blastomycosis clears spontaneously and does not require antifungal therapy. Surgery may be necessary for the drainage of large pulmonary abscesses along with antifungal therapies.
Epidemiology
In North America (the United States and Canada), the disease is primarily endemic, so it has been coined as North American Blastomycosis. However, other continents, including Africa, South America, and Asia, have discovered it. As a dimorphic fungus, B. dermatitidis develops as a mold, forming hyaline branched hyphae as a large single budding yeast at 37 ° C in the laboratory and in human tissues at room temperature in the laboratory and in the community. There are four strains that have been sequenced from Blastomyces dermatitidis, namely: Blastomyces dermatitidis SLH14081, Blastomyces dermatitidis ER-3, Blastomyces dermatitidis ATCC 18188, and Blastomyces dermatitidis ATCC 26199, with the SLH-14081 strain being the most virulent pathogen isolated from samples of immunocompetent persons. In addition, recent research has shown that B. dermatitidis in immunocompromised individuals can cause infection, so it has been identified as an emerging opportunistic pathogen.
Prevention and Control of Blastomyces dermatitidis
As this disease does not spread from person to person, there are no infection control concerns. No vaccine is available and no prophylaxis is recommended there.
Key Notes
- The most commonly used dimorphic medium is brain heart infusion (BHI) agar with blood.
- This organism, Blastomyces dermatitidis comes in risk group III so be careful while processing the specimens
Coccidioides immitis : General Characteristics, Pathogenesis, Clinical Findings, Laboratory Diagnosis, Epidemiology, Prevention, and Control
Introduction of Coccidioides immitis
Coccidioides immitis is a dimorphic fungus with septate hyphae. The fungus causing endemic mycosis –Coccidioidomycosis. It is also known as Valley fever or cocci or California fever, or desert rheumatism, or San Joaquin Valley fever. In endemic areas, the spores of the causative agents are usually found in soil where they are dispersed into the air. It commonly occurs in gardening areas, construction, farming, wind areas. Endemic areas affected are the arid areas of the United States in Arizona, California, Nevada, New Mexico, Texas, Utah, and Northern Mexico. It has two forms. White fluffy mold on most cultural media (Sabouraund dextrose agar) and non-budding spherical form- a spherule, in host tissue. C. Imitis reproduces within mature spherules in the host tissue by forming small endospores. Through the formation of thick-walled barrel-shaped spores, called arthrospores, the fungus is identified by its appearance. The fungus is identified by its appearance by the formation of thick-walled barrel-shaped spores, called arthrospores.
Classification of Coccidioides immitis
Taxonomic Classification
(described by Rixford and Gilchrist in 1896)
Kingdom: Fungi
Phylum: Ascomycota
Class: Euascomycetes
Order: Onygenales
Family: Onygenaceae
Genus: Coccidioides
Species: Coccidioides immitis
Causative agents of Coccidioidomycosis
Dimorphic Fungi, Coccidioides is the causative agent of Coccidioidomycosis. Coccidioides are a genus of dimorphic fungi that exist as mycelia or as spherules of asexual forms and lack the reproduction and structures of the sexual form. The two species of Coccidioides are Coccidioides immitis and Coccidioides posadasii. They are known to cause Coccidioidomycosis in different regions in the endemic areas. These two species are phenotypically similar and can only be identified and differentiated on the basis of molecular tests. Infective spores known as arthroconidia are produced by Coccidioides. During inhalation, the arthroconidia get deposited into the lungs. They then germinate and grow into spherules within the lungs and tissues. Spherules are filled with tiny endospores of about 2µm-5µm, which burst into the tissues releasing the endospores which cause severe disease.
Pathogenesis of Coccidioidomycosis
Transmission: Infection is acquired by dust containing arthrospores. Risk factors of Coccidioidomycosis infection People that are living or traveling into endemic areas where the Coccidioides fungus occurs are at risk of infection. Dust storms containing infected soil fungal spores such as farms or building sites raise the risk of exposure and infection. People who have compromised immune systems are at greater risk of contracting a serious or disseminated disease. These include:
- HIV/AIDS patients
- Organ transplant recipients
- Autoimmune patients and rheumatoid disease patients taking immune-suppressing drugs
- Pregnant women
- Diabetic patients
Virulence factors of the causative agents
Adherence: Autolysis and thinning processes of the spores leave certain barred-shaped cells with the ability to bind to epithelial cells and tissues during mycelial growth and development of the septae. Arthroconidia, light and loosely chained allows them to quickly become airborne and bind to surfaces, and be inhaled by hosts.
Specialization and remodeling: The arthroconidia undergo remodeling when in the host cells shedding off the outer layer of the spore, to form the spherules. The spherules which divide internally through the formation of an internal septate divide the spherules into compartments and each compartment contains several small endospores. In the epithelial cells, alveolar sacs, and alveolar macrophages, a completely impregnated spherule with endospores raptures and releases the endospores. The alveolar macrophages select the endospores that induce an acute inflammatory response as a host response to the endospores due to the aggregation of neutrophils and eosinophils. The endospores can further multiply within the cells and tissues and spread by causing mycelial growth in the tissues.
Antigenic Variation: Two antigens known as coccidiodin and spherulin are generated by Coccidiodes. Coccidiodin is derived from the mycelial cultures of coccidiodes, and broth cultures contain spherulin antigens. They contribute to the immune responses of the fungi.
Clinical manifestations of Coccidioidomycosis
Most of the infections are asymptomatic pulmonary nodules. Many persons develop self-limited influenza-like fever → Valley fever or desert rheumatism (In women reddish, painful, tender lumps known as erythema nodosum or erythema multiforme occur on the legs just below the knees. most commonly located in the front of the legs below the knees, associated with migratory arthralgias, a form of pain the spreads from the joints to other parts of the body. These symptoms are collectively known as desert rheumatism).
- Acute pneumonia
- Chronic fibrocavitatory pneumonia
- Chronic dissemination:
- Bone
- Meninges
- Skin
- Joints Subcutaneous tissue
Some of the major clinical manifestations of coccidioidomycosis include:
- Acute and chronic inflammation is associated with the production of neutrophils and eosinophils in response to endospore exposure in the alveolar sacs and the lung tissues. Neutrophils and eosinophils get attracted to the site where the spherules rupture and release endospores.
- Chronic granulomatous infection occurs when mature spherules do not rupture, which is an indication of Coccidiodes’ control.
- Progressive lung coccidioidomycosis, which is typically chronic with nodules or/and cavity multiplication and enlargement.
- Disseminated coccidioidomycosis, which is crippling and life-threatening; except for pregnant women, it usually affects men than women. Men most affected are the elderly, those with underlying conditions, HIV/AIDS patients. Majorly caused by C. immitis which has estrogen-binding proteins, and elevated levels of estradiol and progesterone stimulate its growth.
- Coccidioides meningitis is a disseminated infection commonly affecting the Central Nervous system and brain of AIDS patients.
Diagnosis of Coccidioidomycosis
Clinical diagnosis of Coccidiodimycosis: Physical examination and history of patients for extrathoracic by chest X-rays that indicate unilateral infiltration, lobar consolidation, nodular infiltrate, cavitation, and hilar and peritracheal adenopathy or mediastinal lymphadenopathy.
Laboratory Diagnosis of Coccidioidomycosis
Specimen: It depends on the site of infections and the most common specimens are sputum, pleural fluid, lesion exudates, cerebrospinal fluid, biopsy
KOH mount: KOH Wet mount and calcofluor stains are used for observation of spherules which are usually 20 to 80 micrometers in diameter, thick-walled, and small endospores of 2 to 4µm for C. immitis.
Culture Characteristics: Culturing Coccidioides in mycological and/or bacterial media produces white to tan cottony colonies within 5-7 days. On SDA, the colonies have hyphae with chains of arthroconidia which independently form hyphal cells. Bacterial media can be prepared with or without antibacterial antibiotics and cycloheximide to inhibit contaminating bacteria or saprophytic molds, respectively. Since arthroconidia are extremely contagious, only in a biosafety cabinet are suspect cultures examined. A complex medium can be used to cultivate and produce spherules of these fungi.
LPCB preparation: It shows hyaline, septate, and thin hyphae and arthroconidia from the plate incubated at 25°C, and also racquet hyphae may occasionally be observed from young cultures. Whereas from the plate incubated at 37°C shows large, round, thick-walled spherules (10-80 µm in diameter) filled with endospores (2-5 µm in diameter). Note: Organism comes at-risk group III and thus strict precautions should be taken.
Histological diagnosis: Using sputum or tissue samples to diagnose thick fungal spherules with a double refractile wall of 80 μm in diameter as shown above image.
Serological Testing
- Eosinophilia, increased ESR or CRP.
- Assay for anti-coccidiodidal antibodies, IgG, and IgM for confirmation
- ELISA for detection of antibodies against the disease or presence of coccidiododial antigens
- Immunodiffusion for detection of IgM and IgG antibodies against coccidioidomycosis
- CFT for IgG for estimation of disease severity, with high titters indication severe disease and low titters, indication less severe disease or decline in severity. CFT also detects the presence of complement-fixing antibodies in the CSF which is an important diagnosis for coccidial meningitis.
- Urine Antigen Test is used in immunocompromised. Patients with significant forms of infection, including pneumonia and disseminated disease.
- Delayed cutaneous hypersensitivity is used for endemic epidemiological research, primarily to detect hypersensitivity to coccidioidin or spherulin, which develops in immunocompetent patients within 10 to 21 days after acute infection. Spherulin, however, is absent in progressive diseases.
Test for Skin: The coccidiosis skin test achieves a maximal induration (about 5 mm in diameter) of 0.1 mL standardized dilution between 24 and 48 hours after cutaneous injection.
Molecular Diagnosis: cDNA probing rapidly identifies fungal growth. PCR is used to test for fungal DNA from samples from the lower respiratory tract.
Treatment of Coccidioidomycosis
Fluconazole or itraconazole can be used to treat mild to severe illnesses. Amphotericin B is treated for serious illnesses. In non-endemic areas where the risk of fungal seeding is low and less hematogenous, patients are treated with less toxic fluconazole. Mild to moderate nonmeningeal extrapulmonary infections are treated with fluconazole or itraconazole are taken orally. Voriconazole can be administered orally or intravenously or treat with oral posaconazole.HIV/AIDS patients with coccidioidomycosis-associated infections use maintained therapy of fluconazole or itraconazole while monitoring the CD4 cell count at about > 250/µl. Meningeal coccidioidomycosis is treated with long-term administration of oral fluconazole. Surgical removal of involved bone to cure osteomyelitis Surgical removal of a lung or pulmonary cavities that cause hemoptysis may be necessary when the disease is diagnosed early to resection the cavity and close pulmonary leaks.
Epidemiology of Coccidioides immitis
Naturally, it exists in many parts of the New World’s soil and air. These are normally dry to semi-arid regions with relatively modest precipitation, mild winters, and prolonged hot seasons. Coccidioidomycosis is typically a disease of both human and non-human inhabitants of these areas; but after entering these areas, tourists may acquire the disease and return home long distances from the endemic areas. Arthroconidia inhalation of C. Immitis leads to an infection that is usually benign, but sometimes serious and sometimes fatal. Recovery from infection or asymptomatic infection leads to reinfection resistance. Exposure to soil indicates that exposure to C. Immitis is more likely for those occupations. The persistence of the organism in the soil means that especially as long as susceptible newcomers continue to penetrate endemic areas, infections will be encountered in the future.
Prevention and Control of Coccidioidomycosis
Near control of risk classes of opportunistic coccidioidomycosis contractors. By minimizing dust, paving highways and airfields, planting grass or crops, and using oil sprays, certain control measures can be accomplished.
Key Notes on Coccidioides immitis
- C. immitis is unique because it produces spherules(30 μm- 60 μm in size), containing endospores(2 μm to 5 μm) in tissue, and hyphae at 25°C.
- Infection of the skin, bones, joints, lymph nodes, adrenal glands, and central nervous system results from the hematogenous spread of the pathogen into the host’s bloodstream.
- C. Immitis is an anaerobic organism that develops spherules in the presence of CO2 quickly.
- The majority of infections with Coccidioides have an incubation period of one to four weeks.
- Without particular treatment, the infection may resolve.
Paracoccidioides brasiliensis: General Characteristics, Pathogenesis, Clinical Findings, Laboratory Diagnosis, Epidemiology, Prevention, and Control
Introduction of Paracoccidioides brasiliensis
Paracoccidioides brasiliensis is a dimorphic fungus that causes Paracoccidioidomycosis, formerly called South American blastomycosis. In parts of Central and South America, this fungus lives. Anybody who lives in or visits areas where it is present can get paracoccidioidomycosis, but it most often affects men who work outdoors in rural areas. Also, the specific habitat of the Paracoccidioides fungus is not precisely known, but it was found in soil near armadillo burrows.
Taxonomic classification of Paracoccidioides brasiliensis
Kingdom: Fungi
Division: Ascomycota
Class: Eurotiomycetes
Order: Onygenales
Family: Ajellomycetaceae
Genus: Paracoccidioides
Species: P. brasiliensis
Morphology of Paracoccidioides brasiliensis
Paracoccidioides brasiliensis is a thermally dimorphic fungus and thus grows in mold form at 25°C and in its yeast form at 37°C.
AT 25°C
Colonies are filamentous, leathery, smooth to wrinkled, woolly, cottony, or glabrous to velvety, with slow development. Within 2 to 3 weeks, the colony matures and its diameter reaches 1 to 2 cm. The front color is cream-white, tan, or brown and the reverse color is brown to brown-yellowish. It produces hyaline, septate hyphae, and aleuriconidia. The hyphae are often sterile and do not sporulate. If present, conidia are oval, unicellular, truncate, and with a broad base and rounded apex. They are located along the hyphae. Arthroconidia and intercalary chlamydospores may also be observed.
AT 37°C
Colonies are yeast-like, white, heaped, wrinkled, or folded. Mold to yeast conversion usually occurs on enriched media, such as brain heart infusion agar, and following 10 to 20 days of incubation. For definitive identification of the fungus, It is fitting to illustrate mold-to-yeast conversion. It develops numerous typical buds that cover the mother yeast cell’s entire surface. A steering wheel resembles this appearance. A narrow neck section connects the daughter cell (bud) to the mother cell. Secondary buds can develop before the bud is detached from the mother cell, forming short chains of yeast cells.
Pathogenesis of Paracoccidioidomycosis
Infection is acquired via the lungs by inhalation of spores from environmental sources. It is most common in humid mountain forests in South and Central America. Males are affected more often than females. It’s possible that female hormones protect women.
Clinical Findings in Paracoccidioidomycosis
Clinical findings depend on the site of involvement as given below.
Pulmonary paracoccidioidomycosis: Most cases have an indolent onset and chronic symptoms such as cough, fever, night sweats, malaise, and weight loss are present in patients. There are characteristic but not diagnostic chest x-rays. It is important to separate the infection from histoplasmosis and tuberculosis.
Mucocutaneous paracoccidioidomycosis: The most typical mucosal sites of infection are the mouth and nose. On the gums, tongue, lips, or palate, painful ulcerated lesions develop and may grow over weeks or months. Palate perforation of nasal septum perforation may occur. Cutaneous lesions around the mouth and nose mostly appear on the face, while widespread lesions may occur in patients with serious infections.
Lymphonodular paracoccidioidomycosis: It is normal for younger patients to have lymphadenitis. The most noticeable manifestation is the cervical and submandibular chains, and lymph nodes may advance to form abscesses with draining sinuses.
Disseminated paracoccidioidomycosis: Paracoccidioides brasiliensis hematogenous spread can lead to widespread disseminated disease, including small or large intestine lesions, hepatic lesions, destruction of the adrenal gland, osteomyelitis, arthritis, endophthalmitis, and meningoencephalitis, or focal cerebral lesions.
Laboratory diagnosis of Paracoccidioides brasiliensis
Specimen: It depends on the site of infection. e.g. in the case of pulmonary paracoccidioidomycosis sputum, pleural fluid, lung biopsy may be taken whereas in mucocutaneous paracoccidioidomycosis cutaneous lesion is preferred.
Direct Examination
Potassium hydroxide (KOH) mount: It shows a large number of yeast cells of P. brasiliensis of about 10–40 µm. Cells usually present as single cells or chains of cells with characteristic multipolar budding.
Culture Characteristics
Paracoccidioides brasiliensis are dimorphic fungi that grow both as molds ( 25°C) and as yeast at 37 ° C. Sabouraud Dextrose Agar (SDA) with yeast extract incubation at 25-30°C for 2 weeks shows mycelial phase.
LPCB preparation
LPCB preparation from the plate incubated at 25°C may show hyaline, septate hyphae, and aleuriconidia while from the plate incubated at 37°C shows multiple, narrow base, budding yeast cells ( steering wheels) as shown above images.
Histological Examination
Following stains are useful for identifications of this fungus and they are-
Hematoxylin and Eosin(H &E) stain: It uses to observe neutrophils and pyogranulomatous reactions due to neutrophilic interactions of the granuloma from the host tissues. Hematoxylin stains the nuclei of cells blue to bluish-purple, and eosin stains the cellular elements in the tissues from pink to red as shown above picture.
Gomori’s methenamine silver stain (GMS): It stains the yeast cell wall deep black while the background is green as shown in the image (B).
Periodic acid-Schiff (PAS) Stain: It stains the yeast cells red with a pink background or light green, identified by the type of counterstain that is used. The histological stains may show multiple, narrow base, budding yeast cells ( steering wheels).
Serological Assay: Immunodiffusion tests and complement fixation tests are useful in the diagnosis of 98% of cases.
Molecular Methods: The sensitivity and speed of traditional methods used in diagnostic mycology have a good potential to complement and boost nucleic acid-based assays. In real-time PCR, the reliability of the internal transcribed spacer (ITS) region for molecular detection of P. brasiliensis has also been shown to be a sensitive technique for rapid paracoccidioidomycosis (PCM) diagnosis.
Epidemiology of Paracoccidioidomycosis
The disease is geographically restricted to Central and South America with high incidence in Brazil, Venezuela, and Columbia. Fungus resides in the soil in an environment that has high humidity. Since 1930, over 15,000 cases of paracoccidioidomycosis have been reported. Many more cases, however, are likely to occur because the disorder is underrecognized. In Brazil, about 80% of the cases reported have occurred. Paracoccidioidomycosis in the United States, where it is not a reportable illness, is possibly uncommon. Scientists predict that fewer than 5 percent of paracoccidioidomycosis patients die from the condition.
Treatment of Paracoccidioidomycosis
Antifungal drugs such as itraconazole and amphotericin B can be used to treat paracoccidioidomycosis. Trimethoprim/sulfamethoxazole, which is also known as co-trimoxazole and has several different brand names, including Bactrim, Septra, and Cotrim, is another drug often used to treat paracoccidioidomycosis. Patients usually need about one year of treatment.
Prevention and Control of Paracoccidioidomycosis
- Ignore the site where Paracoccidioides lives.
- Inform to take the men who work outdoors in rural areas of Central and South America because of being prone to infection.
- If involvement is necessary, wear the protective mask.
- Most cases of paracoccidioidomycosis have been reported from Brazil, Venezuela, Colombia, and Argentina and therefore inform the travelers who are going there to follow safety guidelines.
- To treat paracoccidioidomycosis antifungal drugs are available.
Key Notes on Paracoccidioides brasiliensis
- P. brasiliensis needs to be distinguished from Blastomyces dermatitidis when only single buds are observed and multiple buds are not visible. The buds of Blastomyces dermatitidis are wide-based, unlike Paracoccidioides brasiliensis.
- Between P. brasiliensis and Loboa loboi, cross-antigenicity has been observed.
- Significant characteristics are clinical history, tissue pathology, culture identification with conversion at 37 ° C to the yeast stage.
- WARNING: Blastomyces dermatitidis, Coccidioides immitis, Histoplasma capsulatum, and Talaromyces marneffei cultures pose a significant biohazard to laboratory staff and must be treated in a suitable pathogen handling cabinet with severe caution.
- Conversion from the mold form to the yeast or spherule form and animal pathogenicity have all been used in previous microscopic morphology; however, culture identification is now preferred to minimize exposure to infectious propagules by either exoantigen test or DNA sequencing.
- Mycosis tissue morphology-Blastomycosis: Large broad base unipolar budding yeast cells (8-10 µm). Coccidioidomycosis: Spherules (10-80 µm) with endospores (2-5µm). Histoplasmosis: Small narrow base budding yeast cells (1-5 µm; 5-2um in var. duboisii). Paracoccidioidomycosis: Large narrow base, multi-budding yeast cells (20-60µm). Penicilliosis (Talaromyces marneffei): In this case, there are small, oval to ellipsoidal yeast-like cells (3 µm in diameter). Sporotrichosis (S. schenckii): Small narrow base budding yeast cells (2-5µm).
- Direct examination and culture are the gold standards for the diagnosis of paracoccidioidomycosis.
Further Reading
- Medical Mycology. Editors: Emmons and Binford, 2nd ed 1970, Publisher Lea and Febiger, Philadelphia.
- Rippon’s JW: Medical Microbiology. The pathogenic fungi and the pathogenic Actinomycetes. 3rd ed 1988 Publisher WB Saunder co, Philadelphia.
- Clinical Microbiology Procedure Handbook, Chief in editor H.D. Isenberg, Albert Einstein College of Medicine, New York, Publisher ASM (American Society for Microbiology), Washington DC.
- A Text-Book of Medical Mycology. Editor: Jagdish Chander. Publication Mehata, India.
- Practical Laboratory Mycology. Editors: Koneman E.W. and G.D. Roberts, 3rd ed 1985, Publisher Williams and Wilkins, Baltimore.
- Topley & Wilsons Medical Mycology. Editors: M.T. Parker & L.H. Collier, 8th ed 1990, Publisher Edward Arnold publication, London.
- Textbook of Diagnostic Microbiology. Editors: Connie R. Mahon, Donald G. Lehman & George Manuselis, 3rd edition2007, Publisher Elsevier.
- Mackie and Mc Cartney Practical Medical Microbiology. Editors: J.G. Colle, A.G. Fraser, B.P. Marmion, A. Simmous, 4th ed, Publisher Churchill Living Stone, New York, Melborne, Sans Franscisco 1996.
- https://bio.libretexts.org/Bookshelves/Introductory_and_General_Biology/Book%3A_General_Biology
- MYCOLOGY_SPECIMEN_COLLECTION091614.pdf-Lexington Medical Centre
- Laboratory manual for the diagnosis of fungal opportunistic infections in HIV/AIDS patients
- https://www.ukessays.com/essays/sciences/importance-of-medical-fungi.php
- https://dermnetnz.org/topics/skin-manifestations-of-systemic-mycoses/
- http://www.botany.hawaii.edu/faculty/wong/BOT135/LECT09.HTM
- https://www.ncbi.nlm.nih.gov/books/NBK8125/
- https://www.researchgate.net
- Bailey & Scott’s Diagnostic Microbiology. Editors: Bettey A. Forbes, Daniel F. Sahm & Alice S. Weissfeld, 12th ed 2007, Publisher Elsevier.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC119960
- https://www.microbiologybook.org/mycology/mycology-3.htm
- https://www.sciencedirect.com/topics/medicine-and-dentistry/candida-meningitis
- https://www.britannica.com/science/leukorrhea
- http://www.antimicrobe.org/new/f15.asp
- https://cmr.asm.org/content/20/4/695
- https://www.scielo.br/scielo.php?script=sci_arttext&pid=S0037-86822020000100250
- https://academic.oup.com/cid/article/35/8/909/329202
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2646427/
- https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(15)30058-6/pdf
- https://www.sciencedirect.com/science/article/pii/S1198743X15300586
- https://en.wikipedia.org/wiki/Fusarium
- http://www.himedialabs.com/TD/M455.pdf
- https://www.sciencedirect.com/science/article/pii/S0254629912001573
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC492390/
- https://mycology.adelaide.edu.au/descriptions/hyphomycetes/phialophora/
- https://drfungus.org/knowledge-base/phialophora-species
- http://thunderhouse4yuri.blogspot.com/2011/01/phialophora-varrucosa.html
- https://www.msdmanuals.com/professional/infectious-diseases/fungi/chromoblastomycosis
- https://www.hindawi.com/journals/ecam/2014/957860/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6945559/
- Essentials of Medical Microbiology, Apurba Sankar Sastry.
- https://www.slideshare.net/AnkurVashishtha4/dermatophytes
- https://www.researchgate.net/publication/327393673
- https://mycology.adelaide.edu.au/descriptions/dermatophytes/trichophyton/
- https://www.sciencedirect.com/topics/medicine-and-dentistry/microsporum
- https://www.sciencedirect.com/topics/medicine-and-dentistry/microsporum-audouinii
- https://cmr.asm.org/content/cmr/8/2/240.full.pdf
- https://www.cdc.gov/fungal/diseases/ringworm/definition.html
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5283510/
- https://www.msdsonline.com/resources/sds-resources/free-safety-data-sheet-index/epidermophyton-floccosum/
- https://pedsinreview.aappublications.org/content/33/4/e22
- https://en.wikipedia.org/wiki/Microsporumhttps://mycology.adelaide.edu.au/mycoses/cutaneous/
- https://www.esccap.org/uploads/docs/1afu1a79_0765_ESCCAP_Guideline_GL2_v6_1p.pdf
- http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1413-86702014000200181
- https://cmr.asm.org/content/cmr/8/2/240.full.pdf
- https://watermark.silverchair.com/46-8-811.pdf
- https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/epidermophyton
- https://www.researchgate.net/publication/264553316_Dermatophytosis_Causes_clinical_features_signs_and_treatment
- https://cmr.asm.org/content/cmr/8/2/240.full.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC88888/
- Medical Mycology. Editors: Emmons and Binford, 2nd ed 1970, Publisher Lea and Febiger, Philadelphia.
- Practical Laboratory Mycology. Editors: Koneman E.W. and G.D. Roberts, 3rd ed 1985, Publisher Williams and Wilkins, Baltimore.
- https://www.scielo.br/pdf/aabc/v78n2/a09v78n2.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5674690/
- Sporothrix schenckii and sporotrichosis.Lopes-Bezerra LM, Schubach A, Costa RO An Acad Bras Cienc. 2006 Jun; 78(2):293-308.
- http://www.antimicrobe.org/f12.asp
- https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0007219
- https://www.sciencedirect.com/science/article/abs/pii/S0738081X11002963
- http://www.cdc.gov/fungal/diseases/histoplasmosis /
- http://www.medicinenet.com/histoplasmosis_facts/ article.htm
- Textbook of Microbiology Ananthanarayan & Paniker -9th Edition
- https://en.wikipedia.org/wiki/Histoplasma_capsulatum
- drfungus.org/knowledge-base/histoplasma-species
- https://www.researchgate.net/publication/44576304_Diagnosis_of_Histoplasmosis
- https://www.slideshare.net/shahmanthan24/histoplasmois-mycology-epidemiology-laboratory
- https://www.nepjol.info/index.php/NJB/article/view/26949
- http://www.antimicrobe.org/f02.asp
- https://cmr.asm.org/content/23/2/367
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5657236/
- https://www.cdc.gov/fungal/diseases/blastomycosis/index.html
- https://link.springer.com/article/10.1007/s12281-012-0110-1
- https://www.msdmanuals.com/professional/infectious-diseases/fungi/blastomycosis
- https://www.gov.mb.ca/health/publichealth/cdc/protocol/blastomycosis.pdf
- https://ppdictionary.com/mycology/immitis.htm
- https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/coccidioides-immitis
- https://www.msdmanuals.com/professional/infectious-diseases/fungi/coccidioidomycosis
- https://jcm.asm.org/content/45/1/26https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3702223/
- https://emedicine.medscape.com/article/215978-overview
- https://www.pathologyoutlines.com/topic/lungnontumorcoccidio.html
- https://opentextbc.ca/microbiologyopenstax/chapter/respiratory-mycoses/
- https://pubs.rsna.org/doi/full/10.1148/rg.344130134
- https://nyaspubs.onlinelibrary.wiley.com/doi/abs/10.1196/annals.1406.049
- Practical Laboratory Mycology. Editors: Koneman E.W. and G.D. Roberts, 3rd ed 1985, Publisher Williams and Wilkins, Baltimore.
- https://www.cdc.gov/fungal/diseases/other/paracoccidioidomycosis.html
- https://www.microbiologybook.org/mycology/mycology-6.htm
- https://drfungus.org/knowledge-base/paracoccidioides-species/
- https://mycology.adelaide.edu.au/mycoses/dimorphic/
- https://en.wikipedia.org/wiki/Paracoccidioides_brasiliensis