HIV Test Positive: Introduction, Principle, Procedure, Result Interpretation and Keynotes
HIV Test
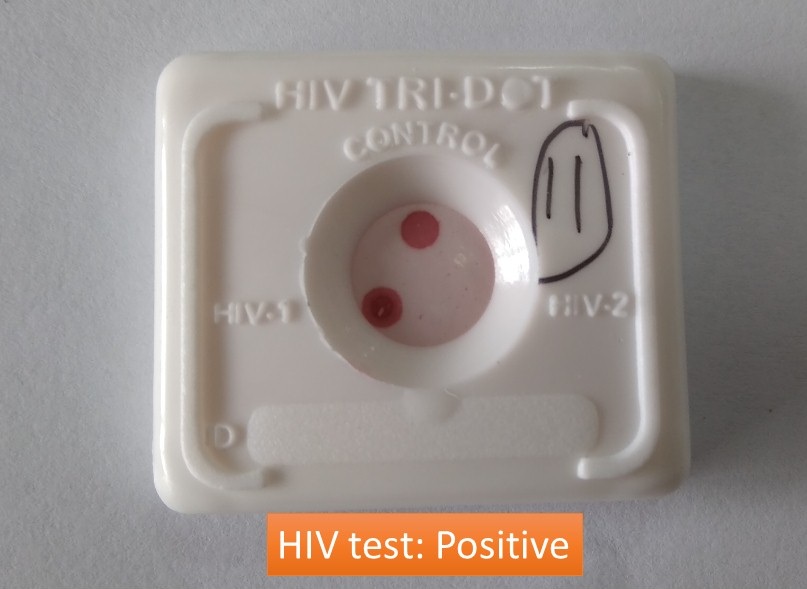
Human Immunodeficiency Virus (HIV) is of two types HIV 1 and HIV 2. HIV 1 is the most widespread type worldwide. HIV 2 is the less prevalent and less pathogenic type and is common in western Africa. HIV 1 rapidly tests positive whereas HIV 2 is negative as shown above image. The HIV test ‘TRI-DOT’ is a visual, rapid, sensitive, and accurate immunoassay for the differential detection of HIV-1 and HIV-2 antibodies (IgM, IgG, and IgA) in human serum or plasma using HIV-1 and HIV-2 Antigens immobilized on an immunofiltration membrane. The test is a screening test for anti-HIV-1 and anti-HIV-2 and is for in vitro diagnostic use only.
Principle HIV Test
HIV antigens are immobilized on a porous immunofiltration membrane. Sample and reagents pass through the membrane and are absorbed into the underlying absorbent. As the patient’s sample passes through the membrane, HIV antibodies, if present, bind to the immobilized antigens. Conjugate binds to the Fc portion of the HIV antibodies to give distinct pinkish-purple DOT(s) against a white background as shown above image.
Test Requirements
- Test Kit contains
- HIV TRI-DOT test device
- Buffer Solution
- Protein-A conjugate
- Sample Dropper
- Extra we need
- Infectious Wastebin
- Gloves
- Timer
- Specimen: serum/plasma
Test Procedure of HIV Test
- Add 3 drops of buffer solution to the center of the device.
- Hold the dropper vertically and add 1 drop of the patient’s sample 50µl (serum or plasma) using the sample dropper provided (use a separate sample dropper for each specimen to be tested).
- Add 5 drops of buffer solution.
- Add 2 drops of Protein-A conjugate directly from the conjugate vial.
- Add 5 drops of buffer solution and read the results.
- Read the results immediately and discard the device considering it to be potentially infectious.
Interpretation of HIV Test Result
- Non-reactive: Only one dot at the control region
- Reactive: If two dots, one for the control and the other for HIV-1 appear, the specimen is reactive for antibodies to HIV-1, as shown above, in the picture. If two dots, one for the control and the other for HIV-2 appear, the specimen is reactive for antibodies to HIV-2. If all three dots, one each for control, HIV-1, and HIV-2 appear, the specimen is reactive for antibodies to HIV-1 and HIV-2.
- Invalid test result: If no dot appears after the test is complete, either with clear background or with complete pinkish/purple background, the test indicates an error. This may indicate a procedural error or deterioration of specimen/reagents or particulate matter in the specimen. The specimen should be tested on a new device.
Keynotes
- Performance characteristics of HIV tri-dot test kits of J. Mitra & Co. Pvt. Ltd have a sensitivity of 100% and a specificity of 100%.
- It is important to allow each solution to soak in the test device before adding the next solution as in the video.
- This HIV Tri-Dot test is based on recombinant DNA technology leading to the differential detection of antibodies to HIV-1 and HIV-2 (if present) in Human Serum or Plasma.
- HIV TRI-DOT has been developed and designed using gp41, C terminal of gp120, and gp36 representing the immunodominant regions of HIV-1 and HIV-2 envelope gene structure respectively.
- The sample found to be reactive by the above screening test must be confirmed
by standard supplemental assay, like Western Blot.
References
- http://jmitra.co.in/download/Procedure/Manual-HIV-Tri-Dot.pdf
- Ajaka Z.L. et.al. (1994). HIV-1/HIV-2 seronegativity in HIV-1 subtype O
infected patients. The Lancet., 343, 1393-1394. - Aman E., Brosius J., (1985): “ATG Vectors” for Regulated High level
Expression of Cloned Genes in E. coli Gene, 40, 183-190. - Guyader, M, Emerman, M, Sonigo, P., Clavel. F, Montaginer L., Alizon M.,
(1987): Genome Organization and Transactivation of the Human
Immunodeficiency Virus Type 2. Nature, 326, 662-669. - Dolt J., Sotz T., Schafer, T., Bergmann, C., (1968): Expression, Secretion, and Processing of Hirudin in E. coli using the Alkaline Phosphatase Signal
Sequence. FEBS Letter, 202, 373-377. - Kan N.C. Franchini G., Wong-Staal F., Dukbois, G.C., Robey. W.G.,
Lautenber, J.A., Papas T.S., (1986): Identification of HTLV-III/LAV SOR
Gene Product and Detection of Antibodies in Human Sera. Science, 231,
1553-1555. - Talha M .S, Salminen T, Chugh D et. al. (2010). Inexpensive designer antigen for Anti-HIV antibody detection with high sensitivity and specificity.
Clinical and Vaccine Immunology.,17:335-341. - Fiebig E, Wright D, Rawal B, Garret P.E et.al (2003).Dynamics of HIV
viremia and antibody seroconversion in plasma donors: implication for
diagnosis and staging of primary HIV infection.,17:1871-1879.
