Principle of Bacterial Motility Test
Organisms are motile due to flagella. A bacterial motility test uses to detect the presence of flagella by bacteria, allowing them to travel in and out of the microscopic field or beyond their initial inoculation in agar. For the wet preparation, a drop of the organism in broth is suspended on a clean and grease-free glass slide, a coverslip is added, and the culture is observed microscopically for motility. Occasionally the organism is incubated in the broth prior to examination whereas, in the tube test, a semisolid motility medium like SIM (Sulfide, Indole, Motility) or MIU (Motility, Indole, Urea) or MIO (Motility, Indole, Ornithine) is inoculated in a straight line down through the center of a tube. Motile organisms will migrate out from the line of inoculation, causing visible turbidity throughout the tube. Non-motile organisms will grow only along the line of inoculation.
Requirements for Bacterial Motility Test
- Organisms tested-Campylobacter; Legionella; enterococci; Enterobacteriaceae; Listeria; Bacillus; other gram-positive rods; non-glucose-fermenting, gram-negative rods; and any other organism where motility is useful for identification
- Broth media like TSB or BHI or Nitrate broth
- A semi-solid medium like SIM, MIU or MIO
- 22- by 22-mm coverslips and microscope slides
- Bright-field microscope or phase-contrast
- Sterile inoculating needles or sticks
- Control strains
Positive Control (PC):Escherichia coli 25922
Negative Control (NC): Klebsiella pneumoniae 13883
Procedure of Bacterial Motility Test
Wet mount preparation
- For Inoculum preparation, use fresh growth from an agar plate and suspend isolated colonies in broth. Use a light inoculum i.e. not visibly turbid.
- It is acceptable to suspend the organism in a small amount of medium for an initial wet mount but follow with incubation of a larger amount of broth media if the result is negative.
- Choosing the medium uses any broth which does not contain carbohydrates and will support the growth of the organisms like BHI, TSB, and nitrate broth.
- Always use a broth for Bacillus spp.
- Use 0.5 ml of BHI or TSB for enterococci.
- Normal Saline can be used for gram-negative rods.
- Use warm sterile tap water for Legionella.
Examination
- Wear gloves and place a small drop of fresh liquid on the center of a microscope slide; add a coverslip. Allow organisms to settle for a minute.
- Focus on 10x objective and observe under high power (40X). For a light microscope, decrease the light by closing the diaphragm. Preferably, using a phase-contrast microscope.
- For all organisms negative for motility by initial wet mount, repeat the wet mount after incubation in broth, or test by tube method below.
- Incubate at 30°C for non-fermenting, gram-negative rods for 24 hours.
- Incubate enterococci and Listeria at 30°C for 2 hours
- Other organisms may be incubated at temperatures optimal for their growth, usually 35°C.
Tube media for Enterobacteriaceae; non-fermenting, gram-negative bacilli or rods; and Listeria
- With a sterile inoculating wire or stick pick an isolated colony and stab the medium straight down through the center to a depth of 1/2 inch for small tubes and 1 inch for larger tubes.
- Incubate as follows:
- At 35°C for the Enterobacteriaceae family for 24 hours.
- At 30°C for non-fermenting, gram-negative rods and enterococci for 24 hours.
- If there is a question regarding a negative result, incubate at 25°C.
- For Listeria and Yersinia, incubate two tubes, one at 35°C and one at 25°C.
Result and interpretation of Bacterial Motility Test
A. Wet mount preparation
Motility test positive: organisms change position with respect to one another
Motility test Negative: organisms do not change position with respect to one another
Brownian movement: It is random jiggling or shaking due to molecular bombardment, where the organisms remain in the same relative position with respect to each other, and should not be mistaken for true motility. Campylobacter displays very active motility which appears as tiny dots darting in and out of the field.
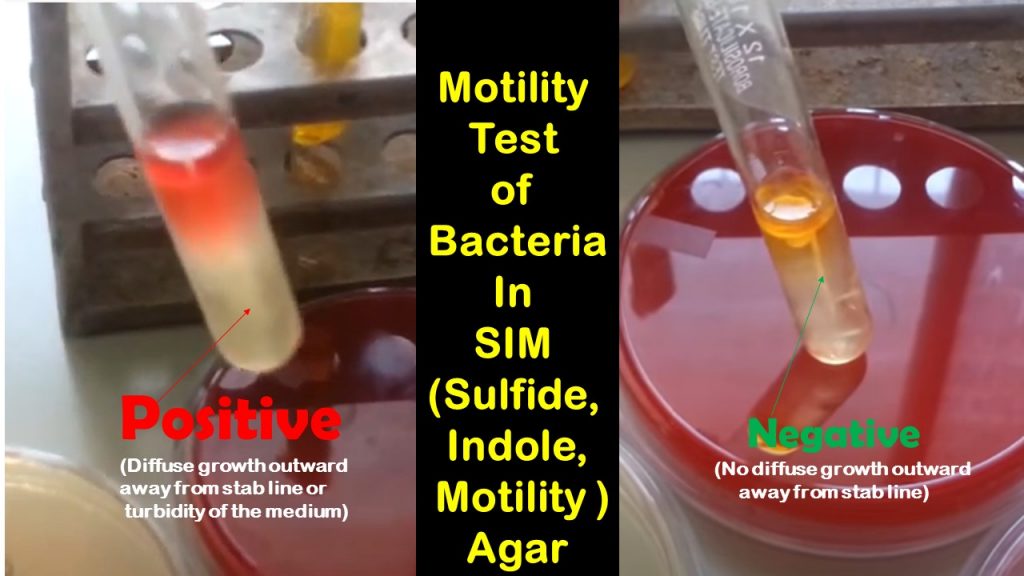
B. Tube media
Motility Test positive: Diffuse growth outward away from the stab line or turbidity of the media
Test negative or non-motile: A clear tube (the same as the uninoculated media) with growth only along the line of inoculation.
Keynotes of Motility Test
- Enterococcus casseliflavus and E. gallinarum are motile.
- Listeria organisms are motile at 25°C but not at 35°C, with a characteristic umbrella-shaped growth at the top of the tube. On wet mount, they exhibit tumbling motility.
- Bacillus spp. should be motile but a lack of motility could indicate Bacillus anthracis.
- Yersinia enterocolitica is motile at 25°C but non-motile at 35°C.
- Acinetobacter species are non-motile.
- Non-fermenting, gram-negative rods, and Enterobacteriaceae vary in their motility.
Limitations of the Motility test
- Motility results for enterococci in MIO have been reported to have poor sensitivity.
- Excessive heat on a microscope slide can affect the test results.
- False-negative reactions may occur if bacterial flagella is damaged due to heating, shaking, or other trauma and such environmental shock will render the organism non-motile.
- Some microorganisms do not express flagellar proteins at 35 to 37°C but do so at 22°C.
- Bacillus species are best tested directly from a fresh agar plate. If a fresh plate is not available, inoculate a plate and incubate for 4 hours. Then perform the wet mount.
- A large number of Enterococcus casseliflavus and Enterococcus gallinarum organisms have been reported as non-motile using some tube motility agar. If a vancomycin MIC is between 4 to 16 µg/ml and the isolate is ampicillin susceptible, but the enterococcus is non-motile, confirm results with the 2 hours broth method or perform the MGP test
Note:-Rice water stool of the cholera-suspected patient is in hanging drop preparation showing darting motility as shown below.
Later on cultivation, TCBS agar has the growth of Vibrio cholera causing infectious disease, cholera.
Vibrio cholerae was isolated on the basis shown below-
Cholera causing bacteria under microscope|| Vibrio cholerae|| Comma shaped bacteria-
Further Readings
- Cowan & Steel’s Manual for identification of Medical Bacteria. Editors: G.I. Barron & R.K. Felthani, 3rd ed 1993, Publisher Cambridge University Press.
- Bailey & Scott’s Diagnostic Microbiology. Editors: Bettey A. Forbes, Daniel F. Sahm & Alice S. Weissfeld, 12th ed 2007, Publisher Elsevier.
- Clinical Microbiology Procedure Handbook, Chief in editor H.D. Isenberg, Albert Einstein College of Medicine, New York, Publisher ASM (American Society for Microbiology), Washington DC.
- Colour Atlas and Textbook of Diagnostic Microbiology. Editors: Koneman E.W., Allen D.D., Dowell V.R. Jr, and Sommers H.M.
- Mackie and Mc Cartney Practical Medical Microbiology. Editors: J.G. Colle, A.G. Fraser, B.P. Marmion, A. Simmous, 4th ed, Publisher Churchill Living Stone, New York, Melborne, Sans Franscisco 1996.
- Textbook of Diagnostic Microbiology. Editors: Connie R. Mahon, Donald G. Lehman & George Manuselis, 3rd edition2007, Publisher Elsevier
- https://catalog.hardydiagnostics.com/cp_prod/content/hugo/miomedium.ht
- https://www.ncbi.nlm.nih.gov/pubmed/17333741
- https://catalog.hardydiagnostics.com/cp_prod/Content/hugo/SIMMedium.html