Kinyoun Stain: Introduction, Principle, Procedure and Result Interpretation
Introduction of Kinyoun Stain
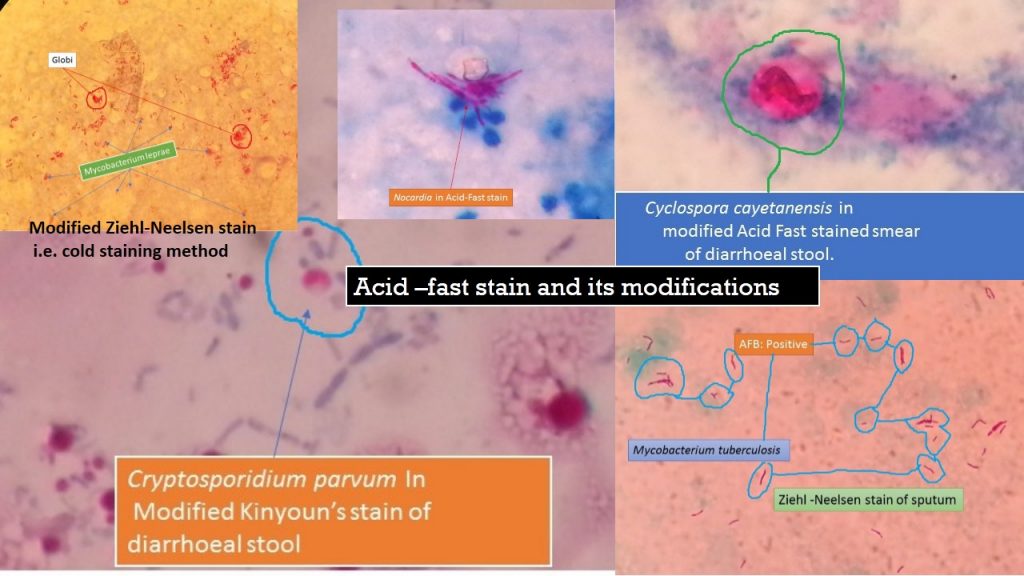
Kinyoun stain is the modified form of acid-fast stain and there is no need for heating in this staining procedure so, called cold method. The stain name is from the surname of developer Joseph J. Kinyoun. It is applicable to stain acid-fast species of the bacterial genera like Mycobacterium and Nocardia and the coccidian parasites Cryptosporidium, Cyclospora cayetanensis and Isospora beli.
Kinyoun Stain Principle
Certain organisms ( e.g. Mycobacterium, Nocardia, and Cryptosporidium) have a waxy lipid called mycolic acid, in their cell walls which allow them to be stained with Acid-Fast better than a Gram-Stain method. The unique ability of these organisms to resist decolorization by acid-alcohol is why they are termed acid-fast. This modified Kinyoun’s stain involves the application of a primary stain (basic fuchsin), a decolorizer (acid), and a counterstain
(methylene blue).
Requirements for Kinyoun Stain
Clean and grease-free slides
Test organism (Here, we have stool from the patient having diarrhea)
Carbol Fuchsin
Sulphuric acid
Methylene blue
Methanol
Tissue paper
Microscope
Reagent preparation
Composition of Carbol fuchsin
Carbol fuchsin: 0.3 gm
95% ethanol : 10 ml
Phenol: 5 gm
Distilled water: 100 ml
Basic fuchsin (solution A)
Dissolve 0.3 g of basic fuchsin in 10 ml of 95% ethanol.
Phenol (solution B)
Dissolve 5 g of phenol crystals in 100 ml of distilled water. (Gentle heat may
be needed.)
Mix solution A with solution B and store at room temperature that is stable for 1 year.
Composition of 5% Sulphuric Acid
Concentrated sulphuric acid: 5 ml
Distilled water: 95 ml
Add 5 ml of concentrated sulfuric acid to 95 ml of distilled water and store it at room temperature. It is stable for 1 year.
Composition of Methylene Blue
Methylene blue: 0.3 gm
Distilled water : 100 ml
Dissolve 0.3 g of methylene blue in 100 ml of distilled water and store at room temperature. It is stable for 1 year.
Kinyoun Stain Procedure
Smear 1 to 2 drops of the specimen on the slide, and allow it to air dry. Do not make the smears too thick (you should be able to see through the wet material before it dries). Prepare two smears. Fix with absolute methanol for 1 minute. Flood slide with Kinyoun’s carbol fuchsin, and stain for 5 minutes. Rinse slide briefly (3 to 5 seconds) with 50% ethanol. Rinse thoroughly with water. Decolorize with 1% sulphuric acid for 2 minutes or until no more color runs from the slide. Rinse slide with water. Drain. Counterstain with methylene blue or brilliant green for 1 minute. Rinse slide with water. Leave for air drying. Examine using low or high dry objectives and finally observe internal morphology, using an oil immersion lens (100X objective ).
Observation of Kinyoun Stained Smears
Observe for acid-fast organisms having cell color is pink to red to deep purple or not. Acid-fast organisms are pink to red to deep purple while non-acid fast organisms or background are blue due to being counter stain methylene blue ( green in case of brilliant green)
Result Interpretation of Kinyoun Stain
Acid-fast organism cell color: pink to red to deep purple
Non-acid fast organism cell color: Blue
Keynotes on Kinyoun Stain
- The concentration of carbol fuchsin is high in this Kinyoun’s modification of acid-fast stain as compare to Ziehl-Neelsen stain (Z-N stain).
- 20% sulphuric acid uses as a decolorizing agent for staining Mycobacterium tuberculosis (Ziehl-Neelsen stain ).
- 5% sulphuric acid uses as a decolorizing agent for staining Mycobacterium leprae ( Modified Ziehl-Neelsen stain i.e. cold staining method),
- 1% sulphuric acid uses as a decolorizing agent for staining Nocardia species, Cryptosporidium, and Isospora oocysts (Kinyoun’s modification of acid-fast stain)
- 0.25% sulphuric acid uses as a decolorizing agent for staining spores.
List of Organisms Having Acid-Fast Structures
Bacterial origin
Mycobacterium smegmatis
Mycobacterium fortuitum
Nocardia brasilnensis
Nocardia caviae
Fungal origin
Parasitic origin
Isospora beli
Other structures
Further Readings
- Isenberg clinical microbiology procedures Handbook
2nd edition. Vol. 2 - Merkell and Voge’s medical parasitology
9th edition. - Parasitology: 12th edition
By K. D. Chatterjee - District laboratory practice in Tropical countries –Part-I.
By Monica Chesbrough. - A textbook of Practical Microbiology -Subhash Chandra Parija
- Manual of Clinical Microbiology. Editors: P.R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover and R. H. Yolken, 7th ed 2005, Publisher ASM, USA.
