Infectious Substance Transportation : Introduction, Triple Packaging and Its Details
Introduction Infectious Substance Transportation
Infectious substance transportation is a complicated networking process in which we have to face to know the nature of infections substance and biological substance, dangerous goods transportation regulations e.g. Air International Civil Aviation Organization(ICAO) and International Air Transport Association (IATA), a working system coordinating with various parties and so on. An infectious substance is defined as a substance containing a viable
microorganism, such as a bacterium, virus, rickettsia, parasite, or fungus, that is known or reasonably believed to cause disease in humans or animals.
With respect to packaging and transport situations, infectious substances include:
- all cultures containing or suspected of containing an agent which may cause
infection; - human or animal samples that contain such an agent in quantities sufficient
to cause infection, should exposure to them occur due to a transport
mishap; - sample(s) from a patient with a serious disease of unknown cause;
- other specimens not included above and designated as infectious by a
qualified person, e.g. a physician, scientist, nurse, etc. Note: This definition is taken from the current UN Recommendations on the
Transport of Dangerous Goods. Prions are not included in this definition
although they are considered to be infectious agents.
Diagnostic specimens: They are defined as any human or animal material including, but not limited to, excreta, blood and its components, tissue and tissue fluids collected for the purposes of diagnosis, but excluding live infected animals.
The objective of this topic includes-
- Differentiate between infectious substances and biological substances for the purposes of transportation
- Describe the types of packaging and documentation appropriate to each
- Be aware of special requirements for other dangerous goods and import/export permits
- Transportation of infectious substances and biological substances
√ Regulation on the transport of dangerous Goods – UN committee of experts on dangerous Goods, UN Model
√Technical institution for safe transport of dangerous Goods- Air International Civil Aviation Organization(ICAO)
√Dangerous Goods regulations – International Air Transport Association (IATA)
- A working system coordinating with various parties
√Agreements and procedures for the shipment of infectious substances are developed with local customs, air transport, and postal authorities. It includes emergency plans in the event of an emergency.
√ Continual liaison between all parties must be maintained to reflect changes in personnel, regulations, local conditions, and status of air carriers.
Classification of Infectious Substances
The three proper shipping names for infectious substances are:
- Infectious substances, affecting humans, UN 2814
- Infectious substances affecting animals, UN 2900
- Biological Substances Category B, UN 3373 (This does not require a Dangerous Good Declaration to ship.)
Categories of Infectious Substances
Category A: Capable of causing permanent disability, life-threatening or fatal disease in otherwise healthy humans or animals. Assigned to UN 2814 if cause disease in humans or animal. Assigned to UN 2900 if cause disease only animals. Examples of infectious substances included in Category A UN 2814 Infectious substances affecting humans are the only culture of various organisms like Bacillus anthracis , Brucella abortus, Brucella melitensis, Brucella suis, Burkholderia mallei, Burkholderia pseudomallei , Chlamydia psittaci (avian strains), Clostridium botulinum, Coccidioides immitis , Coxiella burnetii, Dengue virus, Eastern equine encephalitis virus, Escherichia coli-verotoxigenic and Francisella tularensis. whereas any particle of the Ebola virus, Crimean-Congo hemorrhagic fever virus is included in this category.
Category B
An infectious substance that does not meet the criteria for inclusion in category A. They are considered to be less risk and assigned to UN 3373. However, culture samples are assigned to UN 2814 or UN 3373. Culture is the result of a process of intentionally propagating pathogens.
Biological products
That product is derived from living organisms and used in the prevention, treatment, or diagnosis of disease in humans or animals or for development, experimental, or investigation purposes. Not limited to finished or unfinished products such as vaccines.
Patient Specimens
Human and animal materials collected directly from humans or animals are being transported for research, diagnosis, investigational activities, disease treatment, and prevention.
Other Definitions
Genetically modified (GM)- The are micro-organisms and organisms in which genetic material has been purposely altered in a way that does not occur naturally. Assigned UN 3245 UN 3245. Medical or clinical waste and they are waste derived from the medical treatment of animals or humans or from bio-research. Assigned to UN 2814 or UN 2900.
Packaging, labeling, and documentation for transport of Infectious Substance
- Packaging requirements are determined by the UN and are contained in ICAO and IATA regulations
- Packaging instructions 602 and 650.
- The requirements are subject to change and upgrade by these associations.
- UN-approved packaging systems are available commercially.
Packaging Requirements for Infectious Substances
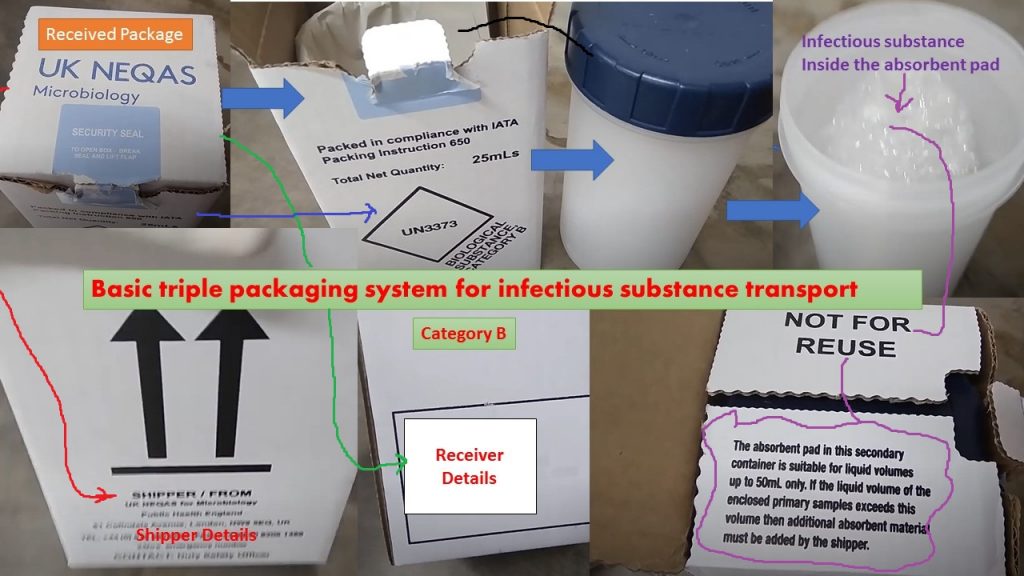
Triple Packaging
Primary receptacle
A labeled primary watertight, leak-proof receptacle containing the specimen. The receptacle is wrapped in enough absorbent material to absorb all fluid in case of breakage.
Secondary receptacle
A durable, water-tight, leak-proof receptacle containing primary receptacle.
Outer shipping package
Rigid, bearing forms, permits, etc.
Category B, 650 package
- package UN 3373
- No biohazard label
Category A 602 package
- Labels: UN 2814
- UN 2900 Biohazard
Triple Packaging
UN Certified Bio Bottle
Packaging Requirement for Biological products
- In accordance with the requirements of appropriate national authorities.
- They may have special licensing requirements.
- Basic triple packaging meets IATA packaging Instruction 650.
- May contain up to 500 ml not to exceed 4 liters.
- Labels marked with diamond with UN 3373 and Biological substance, category B.
Transport Planning
It is the responsibility of the sender to ensure the correct designation, packaging, labeling, and documentation of all infectious substances and diagnostic specimens. The efficient transport and transfer of infectious materials require good coordination between the sender, the carrier, and the receiver (receiving laboratory), to ensure that the material is transported safely and arrives on time and in good condition. Such coordination depends upon well-established communication and a partner relationship between the three parties. All have specific responsibilities to carry out in the transport effort.
The sender
- makes advance arrangements with the receiver of the specimens including
investigating the need for an import permit; - makes advance arrangements with the carrier to ensure:
√that the shipment will be accepted for appropriate transport
√that the shipment (direct transport if possible) is undertaken by the most
direct routing, avoiding arrival at weekends; - prepares necessary documentation including permits, dispatch, and shipping documents;
- notifies the receiver of transportation arrangements once these have been
made, well in advance of the expected arrival time.
The carrier
- provides the sender with the necessary shipping documents and instructions for their completion;
- provides advice to the sender about correct packaging;
- assists the sender in arranging the most direct routing and then confirms the routing;
- maintains and archives the documentation for shipment and transport;
- monitors required holding conditions of the shipment while in transit;
- notifies the sender of any anticipated (or actual) delays in transit.
The receiver
- obtains the necessary authorization (s) from national authorities for the
importation of the material; - provides the sender with the required import permit(s), letter(s) of
authorization, or another document (s) required by the national authorities; - arranges for the most timely and efficient collection on arrival;
- immediately acknowledges receipt to the sender.
Shipments should not be dispatched until:
- advance arrangements have been made between the sender, carrier and
receiver - the receiver has confirmed with the national authorities that the material
may be legally imported - the receiver has confirmed that there will be no delay incurred in the
delivery of the package to its destination. - Detailed information on response and emergency safety measures in transport-associated accidents can be found in Laboratory Biosafety Manual, Second edition (1993)Geneva: World Health Organization.
Specimen packaging and transport for “severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
Sample should be safely packed in Triple container packing and should be transported under a cold chain to the reference laboratory with prior intimation. The packaging consists of three layers as follows.
- Primary receptacle: A labeled primary watertight, leak-proof receptacle containing the specimen. The receptacle is wrapped in enough absorbent material to absorb all fluid in case of breakage.
- Secondary receptacle: A second durable, watertight, leak-proof receptacle to enclose and protect the primary receptacle(s). Several wrapped primary receptacles may be placed in one secondary receptacle. Sufficient additional absorbent material must be used to cushion multiple primary receptacles.
- Outer shipping package. The secondary receptacle is placed in an outer shipping package which protects it and its contents from outside influences such as physical damage and water while in transit.
- Specimen data forms, letters, and other types of information that identify or describe the specimen for “testing of SARS-CoV-2– Severe Acute Respiratory Disease ” and also identify the shipper and receiver should be taped to the outside of the second receptacle.
Key Notes
- The safe expeditious shipment of diagnostic specimens and infectious agents begins with the development of a working system between the sender, postal or airline officials, customs, and the receiving laboratory.
- When such mutual agreements and understandings are made, common major encountered problems are prevented before they occur.
- Laboratory personnel has the responsibility to become familiar with the appropriate transport regulations for the microorganisms and specimens with which they work.
Further Readings
- https://www.who.int/csr/emc97_3.pdf
- https://www.powershow.com/viewht/44a696 YjU3Y/Packaging_and_Shipping_Laboratory_Specimens_powerpoint_ppt_presentation
- http://www.safetyway.es/en/containers-for-transport-of-diagnostic-samples/medimail/triple-packaging.html
- https://ncdc.gov.in/WriteReadData/l892s/50471431021580628750.pdf
- https://www.iata.org/en/publications/store/infectious-substances-shipping-guidelines/
- https://www.biosafety.be/content/safety-measures-transport-gmos-andor-pathogens
- https://apps.who.int/iris/bitstream/handle/10665/325884/WHO-WHE-CPI-2019.20-eng.pdf?ua=1
- https://www.icao.int/publications/Documents/guidance_doc_infectious_substances.pdf
