HPV Genes detection using Real-Time PCR: Introduction, Principle, Test Requirements, Procedure, Result Interpretation and Keynotes

Introduction of HPV Genes Detection
Prior to dealing with HPV Genes Detection, let’s discuss the virus. Human papillomaviruses (HPV) are small double-stranded (ds) DNA viruses that belong to the family of Papillomaviridae. having a circular genome of the size of approximately 7.9 kilobases. There are more than 100 types of HPV that have been identified, and so far among them. Certain HPV types are called high-risk HPV (hrHPV) like HPV 16 and 18. They are associated with the induction of mucosal lesions that can progress to malignancy. The viral genome contains early (E) and late (L) genes, which encode proteins necessary for the early and late stages of the HPV life cycle. The E6 and E7 gene products of hrHPV types have carcinogenic properties and are necessary for the malignant transformation of the host cell.
Malignant progression is often associated with viral integration into the host cell genome. Integration results in the interruption of the viral genome in a region that may extend from the E1 to the L1 open reading frame (ORF). This may have consequences for PCR-mediated amplification of viral DNA in these regions. As initiation and maintenance of the transformed phenotype depend on the continuous expression of the viral oncoproteins, the viral E6/E7 region is invariably retained in integrated viral genomes in cervical cancers. The QIAscreen HPV PCR Test targets a conserved region within the E7 gene.
HPV gene detection using real-time PCR is both a screening and confirmatory test of Human papillomaviruses that is responsible for the malignant transformation of the host cell. The human papillomaviruses (HPV) nucleic acid test kit is a multiplex, real-time PCR test intended for the qualitative detection of nucleic acid from the human papillomaviruses in the specimens such as a cervical specimen(scrape) sample or self-collected vaginal brush specimens or self-collected cervicovaginal lavage specimens in respective preservative solutions of HPV 16 gene, HPV 18, HPV Others and human housekeeping gene β-globin.
Principle of HPV Genes Detection Using Real-Time PCR
The QIAscreen Human Papillomavirus (HPV) PCR Test is a multiplex, real-time PCR-based test and it is directed against the E7 gene of 15 hrHPV types that uses fluorescent probes for the detection of one or more accumulating PCR products/ amplicons. During each PCR cycle, the fluorescent signal increases in a logarithmic manner, causing an amplification curve. After completion of the reaction, the result will be determined through analysis of the cycle threshold (Ct) of each channel. The multiplex format permits the simultaneous detection of four different fluorescent dyes per reaction, with each fluorescent dye representing different targets. HPV 16, HPV 18, 13 other hrHPV types (as a pool), and the human β-globin gene are the four different targets.
This assay separately detects HPV 16, HPV 18, and the pool of 13 other hrHPV genotypes. Moreover, the kit is designed with the human β-globin gene is a housekeeping gene and it is used for monitoring sampling, extraction, specimen addition, amplification, and other related processes as an internal standard to effectively prevent false positive and false negative results, so as to ensure the specificity and accuracy of the test results.
Requirements for HPV Genes Detection Using Real-Time PCR
- Extracted DNA/template from the specimen-The specimen may be either a cervical specimen(scrape) sample or self-collected vaginal brush specimen or self-collected cervicovaginal lavage specimens in respective preservative solutions.
- The Human Papillomavirus (HPV) nucleic acid test kit contains-
- Master Mix
- Human Papillomavirus (HPV) positive quality control
- Negative quality control
Extra we need-
- Lab coat (apron), disposable gloves, and protective goggles.
- Dedicated pipets (adjustable) for PCR (1–10 µl; 10–100 µl)
- Dedicated filter-plugged sterile DNAse-free pipette-tips
- Disposable gloves
- Benchtop centrifuge
- Vortex mixer
- Refrigerators
- Thermocycler (PCR machine)
- PCR cabinet
- Polymerase chain reaction tubes with cap (0.1 ml Strip Tubes and Caps)
- PCR tube holder
Procedure of HPV Genes Detection
All reagents must be thawed completely before use. After mixing centrifuge them at 6000 rpm for a few seconds before use.
Nucleic acid (DNA) Extraction
The nucleic acid extraction kit will be used for specimen extraction. The extracted nucleic acid /template will be tested immediately, or it shall be stored at -20 °C. No extraction is required for this test kit (Qiagen) for positive and negative controls. Use recommended commercial nucleic acid extraction kit.
Mater Mix Preparation
The volume of the master mix per reaction is multiplied by the number of samples, which includes the number of the control and samples prepared. For reasons of unprecise pipetting, always add an extra virtual sample. Mix completely and then spin down briefly with a centrifuge/short spinner.

Template Addition
Pipet 15 µl Master mix with micropipette of sterile tips to each of the Real-Time PCR reaction plates /tubes. Separately add a 5 µl template (negative control and nucleic acid extracted from specimen, positive control) to different reaction plates /tubes. Immediately close the plates/tubes to avoid contamination, and centrifuge the mixture instantaneously in order to collect Master Mix in the bottom of the reaction tubes.

Real-Time PCR Amplification
Put the complete PCR reaction tube into the fluorescent quantitative PCR analyzer (thermocycler-Rotor-Gene Q MDx 5plex HRM (CA) or Rotor-Gene Q bur here used QuantStudio 5 Real-Time PCR), set the positive quality control detection hole, and set the specimen name.
Total reaction system: 20 µl
The cycle parameter setting is shown in the table.
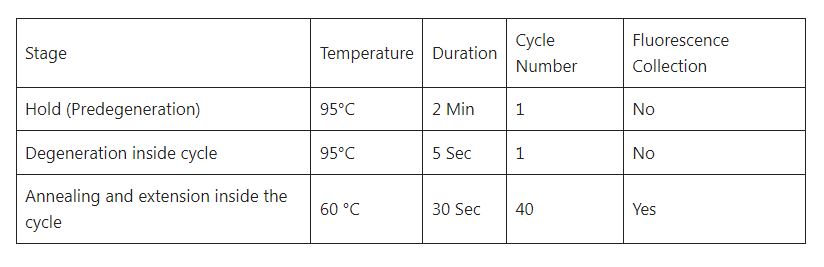
Note-There is no reference fluorescence for the kit, and select “None” for the quencher; save the file after setting, and run the reaction program.
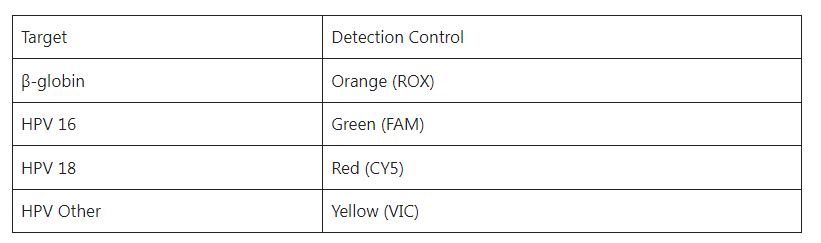
Fluorescence detection channel selection: FAM, CY5, VIC, and ROX channels are selected for detection: FAM is the HPV 16 gene indicator channel, CY5 is the HPV 18, VIC is the HPV Others indicator channel, and ROX is the internal control β-globin indicator channel.
Target and channel settings
Result Analysis for HPV Genes Detection
After the reaction is completed, the system saves the results automatically, and the baseline and the threshold of each detection are adjusted according to the image after analysis. Click Analysis for the analysis and make the parameters meet the requirements in quality control and then check the result of each unknown specimen.
Quality Control
Negative quality control: No exponential increase in any channel amplification plot or Ct≥40.
Positive control: The amplification plots of the four channels, i.e FAM (Ct<30), CY5 (<30), VIC (<32), and β-globin (<29). Note-The above two items must be satisfied in the same test at the same time, or the test is deemed invalid and the detection will be carried out again.
Cut–off (CO ) Value or Reference Interval
Based on the clinical specimen test results the ROC (receiver operating characteristic) curve method is adopted. To determine the cut-off ( CO) value of these kits as follows.
- It is FAM positive if the FAM channel amplification plot shows an exponential increase with Ct <36.
- It is CY5 positive if the CY5 channel amplification plots an exponential increase with Ct<36.
- It is VIC positive if the VIC channel amplification plots an exponential increase with Ct<33.5.
Interpretation of Test Results for HPV Genes Detection
- In each experiment, it’s necessary to test negative quality control and HPV positive quality control, and the results can only be determined when the results meet the quality control requirements.
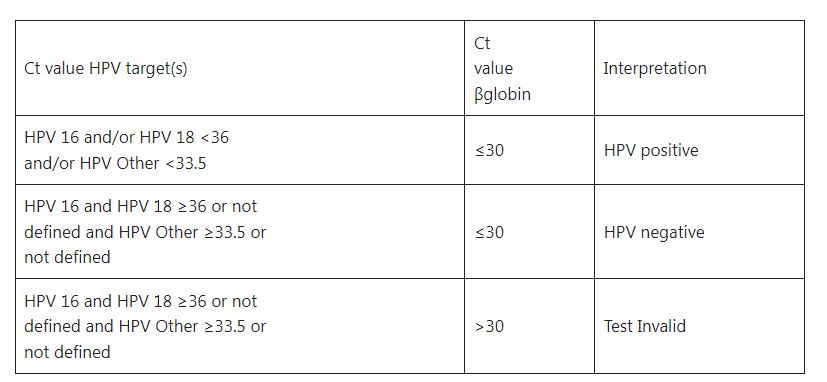
- The criterion for a positive specimen: It can be reported as a positive specimen when the FAM, CY5, VIC, and also β-globin channels are positive.
- The criterion for a negative specimen: It can be reported as a negative specimen when the channel β-globin is positive while FAM, CY5, and VIC channels are negative.
- When the β-channel is negative, the test results of the specimen are invalid. It may be caused by sampling, extraction, specimen addition, amplification, and other processes. It is recommended to conduct the test after pre-extraction or after re-sampling.
Result and Interpretation Table
Keynotes on Human Papillomavirus (HPV)
- The storage time shall not exceed three days, and repeated freeze-thaw cycles shall be avoided.
- The human housekeeping gene (β-globin/RNaseP) is used as the sample control determining both the quality of the sample DNA and the presence of potential inhibitory substances.
- Reporting format of HPV DNA Detection is as follows-
Human Papillomavirus (HPV) DNA PCR and Genotyping
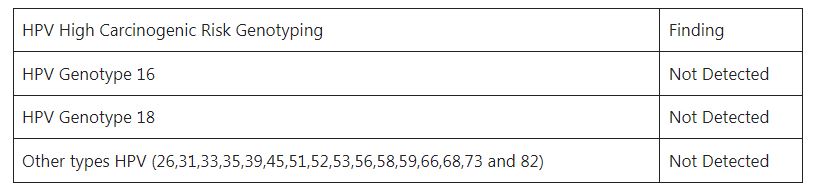

Interpretation
- A positive result indicates the presence of the Human Papillomavirus (HPV).
- A negative result indicates the viral concentration in the sample is lower than the detection limit of the kit and thus in this scenario, the infection can not be excluded.
- Improper collection of specimen handling, transportation, and storage may result in a false negative.
- The PCR reaction targets the L1 loci of the HPV genome which is highly specific that is common in all types of Human Papillomavirus (HPV).
- The results are only for clinical reference and they should be analyzed in combination with clinical signs and symptoms. The medical history, treatment response, and results of other laboratory examinations are very important.
- These results are based on the real-time PCR amplification by QuantStudio 5 Real-Time PCR system.
Note: The report should be on the organization sheet with the signature/s and NMC/NHPC registration number of laboratory personnel who are involved in testing and reporting.
Further Reading
- https://www.cdc.gov/std/hpv/stdfact-hpv.htm
- https://www.who.int/news-room/fact-sheets/detail/cervical-cancer
- QIAscreen HPV PCR Test Instructions for Use (Handbook)
- https://www.mayoclinic.org/diseases-conditions/hpv-infection/symptoms-causes/syc-20351596
- https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-vaccine-fact-sheet
- https://www.journals.elsevier.com/papillomavirus-research/recent-articles
